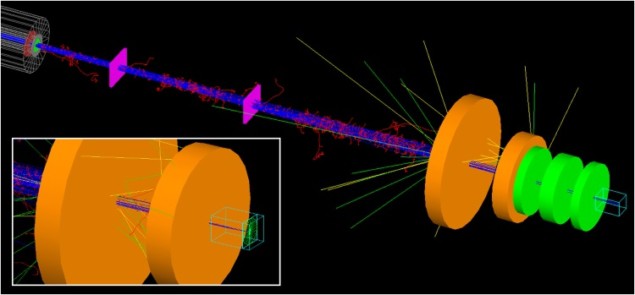
Improvements in imaging technologies have enabled earlier detection of cancers, creating a need to treat increasingly smaller targets. For proton therapy, however, as the field size decreases below 1.0 cm diameter, multiple Coulomb scattering (MCS) causes broadening of the proton beam. The ensuing beam degradation is typically overcome by using additional treatment beams. But researchers at Loma Linda University are investigating another option: magnetic focusing of protons.
The idea is to counteract MCS by focusing the proton beam immediately before it enters the tissue. Focusing is achieved using a triplet of quadrupole magnets, which produce beams with the near-circular cross sections required to irradiate small spherical targets.
“The main clinical application of magnetic focusing will be irradiation of targets less than 1 cm diameter,” explained senior author Andrew Wroe. “These targets are seen with some regularity in intracranial radiosurgery, and early detection is leading to targets of this size in a number of other anatomical regions.”
The Loma Linda team has now used Monte Carlo simulations to investigate the dosimetric impact of this approach (Phys. Med. Biol. 63 055010).
MC modelling
Wroe and colleagues used Monte Carlo simulations to model protons travelling through a beam line and delivered to a water phantom. They chose a proton energy of 118 MeV to match the range in water (10 cm) commonly used for intracranial radiosurgery. Magnet parameters were selected to match commercial rare-earth permanent magnetic materials.
The researchers compared unfocused collimated proton beams (UNF) with proton beams focused using the magnet triplet (MF3). To do this, they performed baseline simulations with 5, 6, 7, 8, 9, 10 and 11 mm diameter UNF beams, plus matched MF3 beams. Matching was achieved by varying the magnetic field gradient of the triplet (100, 150 or 200 T/m), inter-magnet separation and initial beam diameter. In some cases, more than one combination of initial beam diameter and magnet gradient matched a UNF beam, resulting in 11 investigated MF3 configurations.
All MF3 beams produced spots at the Bragg peak depth with low eccentricity and full-widths at the 90% dose contour from 2.5 to 5 mm. In the 10 cases where the MF3 initial beam diameter was greater than the UNF beam diameter, the focused beams had 16-83% larger peak-to-entrance dose ratios, and 1.3 to 3.4-fold increases in dose delivery efficiency, compared with their matched UNF beams.
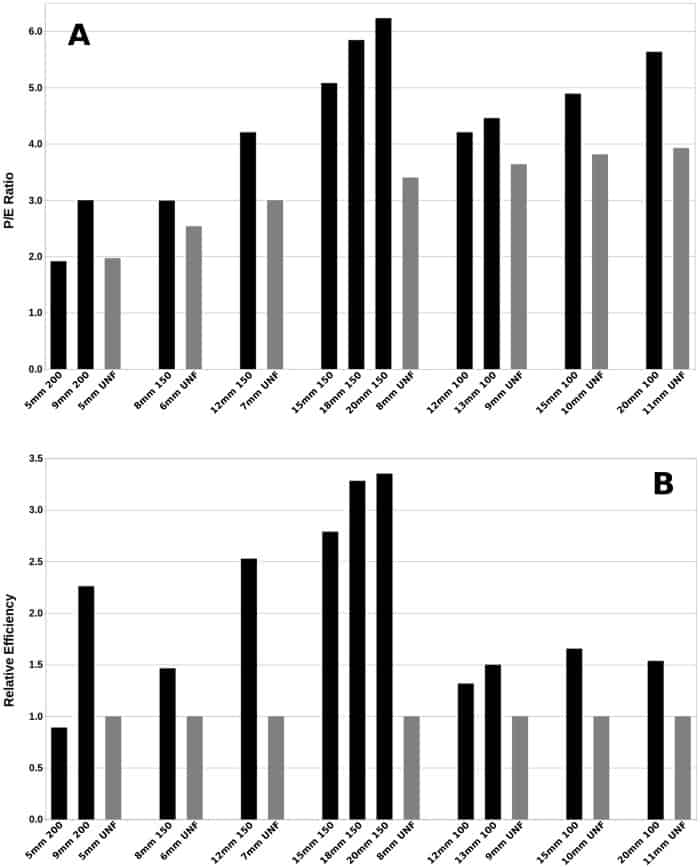
An increased peak-to-entrance dose ratio implies a reduced entrance dose, which for head lesions, translates to a lower dose to the cortex, subcortical regions and scalp. Increased delivery efficiency, meanwhile, could reduce treatment times and increase patient throughput.
“Focused proton beams have the potential to irradiate small radiosurgery targets with lower entrance dose, fewer treatment beams and shorter treatment times compare to current beam delivery methods,” explained lead author Grant McAuley.
“With the shift towards shorter treatments (such as SBRT and SRS courses) for a wider range of clinical sites, the dose rate enhancement potential of magnetic focusing could lead to not only more conformal, but also shorter treatments, which is an important consideration as dose per fraction increases,” Wroe noted.
In cases with more than one MF3 configuration matching a particular UNF beam, certain configurations exhibited superior beam properties. For example, peak-to-entrance dose ratio and efficiency tended to increase with larger magnet gradients and larger initial MF3 beam diameter.
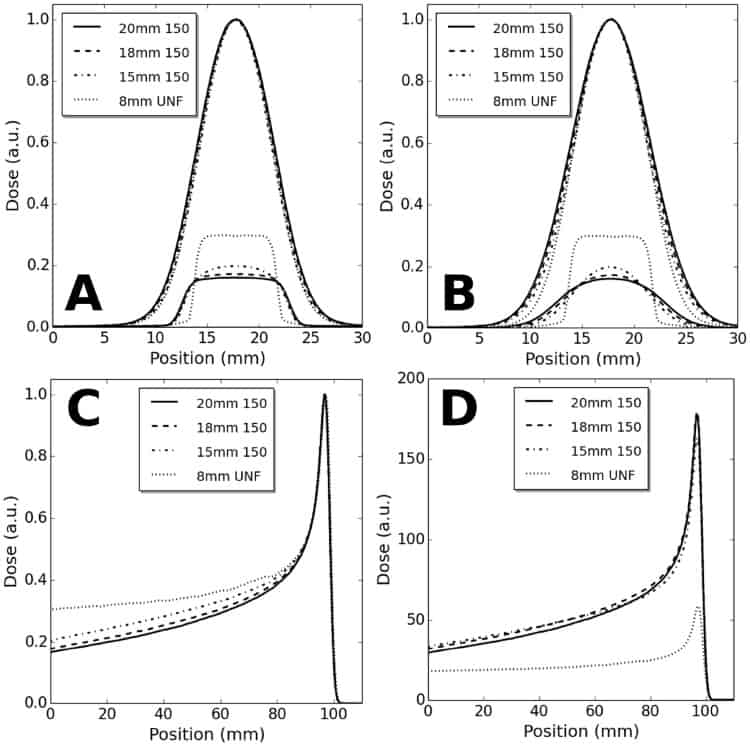
Larger beam diameters, however, generally increase the integral dose. For MF3 beams focused with magnet gradients of 150 T/m and 100 T/m, integral doses were 0-14% and 10-20% larger than their UNF counterparts, respectively, although focusing tended to shift dose from the 80%-20% dose range to below 20% of reference dose.
They researchers note that, for a given magnet gradient, it may be possible to reduce integral dose by using a smaller initial beam diameter, without significant performance loss. They also emphasize that this increased integral dose is based on a single-beam comparison, whereas the increased peak-to-entrance dose ratio may enable removal of at least one treatment beam, greatly reducing the overall integral dose.
Into the clinic
For clinical implementation, focusing magnets made of rare-earth permanent magnetic materials can be manufactured at reasonable costs. Such magnets require neither power nor cryogens and could be easily incorporated into existing proton treatment nozzles.
The researchers envision that they will optimize the focusing system for 3-4 specific target field sizes, such that 3-4 magnetic focusing cones can be employed for proton treatment. The cones would be prescribed during treatment planning and when a specific focusing diameter is required, the appropriate cone will be selected and used in the treatment.
“The optimization will involve balancing parameters including treatment efficiency, peak-to-entrance dose ratio and penumbra. Once this optimization is completed and the cones manufactured, it will not need to be repeated for each patient or use of the system,” Wroe explained. “We are currently working on the prototype system and hope to present the experimental results at the AAPM Annual meeting this year.”



