
Proton therapy offers dosimetric advantages over conventional photon radiotherapy, but is far less forgiving to tumour localization uncertainties, anatomy changes and set-up variations. In proton treatments of lung tumours, for example, a recent study reported that daily imaging and frequent treatment adaptation should be mandatory for a high proportion of patients.
Currently, image guidance in proton therapy is based on 2D kilovoltage (kV) imaging, which lacks soft tissue contrast. Some newer proton machines incorporate cone-beam CT for 3D image guidance, but a wide variation in Hounsfield units (HU) can result in unacceptable uncertainties in calculations of relative stopping power ratio.
Instead, a team at Washington University School of Medicine propose the use of a mobile helical CT (mCT) scanner to provide 3D volumetric imaging for image-guided and adaptive proton therapy (Radiother. Oncol. 10.1016/j.radonc.2018.08.021).
“The mCT provides superior imaging quality with a larger field-of-view than cone-beam CT,” explains first author Baozhou Sun. “It does not need rails installed in the treatment room and is not mechanically constrained. In addition, it can be shared in multiple treatment rooms for a more cost-efficient solution.”
System comparisons
Sun and colleagues integrated a large-bore, 32-slice BodyTom mCT into the clinical workflow for image-guided adaptive proton therapy, using a patient couch that rotated 90° between treatment and imaging positions.
The researchers first compared the imaging quality of the mCT with that of a helical CT scanner used for proton simulation and planning. Overall, image quality parameters were comparable for the two scanners, for both abdomen and pelvis scans. They note that maximum and mean HU deviations were slightly smaller for the mCT.
A major challenge when employing an mCT without rails is accurate alignment of the imaging isocentre with the treatment (and room) isocentre. To achieve this, the researchers attached a stereotactic reference frame to the treatment couch. The frame contains four radiopaque fiducials for detection by the mCT, and four infrared markers visible to a ceiling-mounted camera calibrated to the room isocentre.
During scanning, instead of couch motion, the CT scanner moves across the floor. Thus, any vibration or mis-calibration of the CT gantry motion could result in poor image quality. To evaluate the mCT’s geometric accuracy, the team scanned a spiral phantom with 25 high-contrast markers and compared coordinates from the planning CT and the mCT. While the mCT performed slightly worse, its mean and maximum deviations were all less than 1 mm. It also achieved a geometric accuracy comparable to that of any gantry-mounted cone-beam CT.
The researchers next compared the localization accuracy of the mCT with that of an orthogonal kV imaging system installed in the treatment room and aligned with the treatment isocentre. They found a maximum deviation of 0.3 mm between mCT and kV localization.
To evaluate the accuracy of the relative stopping power ratio, the researchers compared water equivalent distance (WED) on scans of brain and lung phantoms. Absolute differences between WEDs measured on the mCT and planning CT were 0.8 ± 0.6 mm and 1.3 ± 0.9 mm, for brain and lung phantoms, respectively. Maximum differences occurred at relatively large proton ranges, but even for the worst-case scenarios, WEDs agreed within 2.3%.
The researchers also examined the proton dose distribution to a target volume delineated on the planning CT and copied to the registered mCT image. Overall, dose differences between the two were small, with the largest discrepancies observed at distal range locations with high dose gradients. A 3D gamma analysis (3%/3 mm) showed passing rates of 95.1% and 95.3%, for the brain and lung cases, respectively.
Adaptive planning
Since the end of 2016, the mCT has been used in clinical proton treatments at Washington University in St. Louis. To demonstrate the mCT’s use in guiding plan adaptation, the researchers presented two example patient cases. In both, mCT images were acquired prior to treatment delivery and registered with the planning CT images to evaluate any changes in target coverage or dose to organs-at-risk.
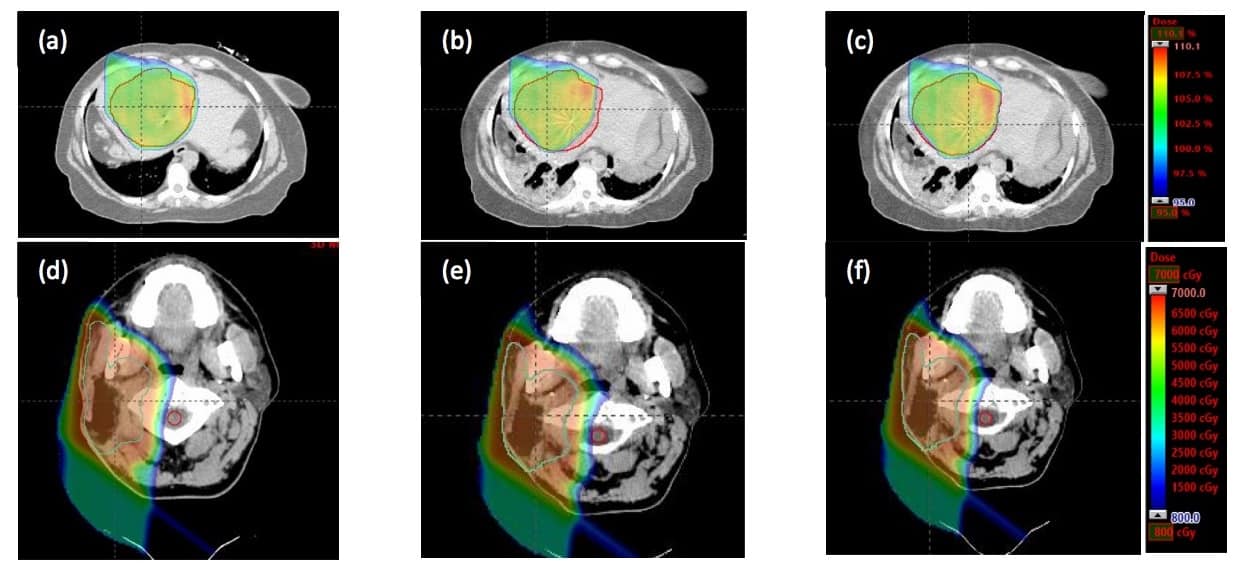
For a liver SBRT case, comparing dose distributions on the planning CT and mCT revealed a reduction in the target volume receiving 50 Gy from 98.1% to 92.4%, caused by changes in the abdominal air cavity. Replanning by adding 1.5 cm range on one beam increased this volume to 98%.
In the second case, a head-and-neck cancer patient had previously undergone radiotherapy and was retreated with proton therapy due to tumour recurrence. The mCT images revealed a maximum spinal cord dose of 19.6 Gy, twice the dose seen on the planning CT and exceeding the maximum specified dose of 10 Gy. Revising the treatment plan by reducing the beam range slightly reduced the cord dose to 9.8 Gy.
“We are in the process of installing a second compact proton machine with pencil-beam scanning capability,” Sun tells Physics World. “The mCT has the potential to provide online adaptive proton therapy for intensity-modulated proton therapy.”



