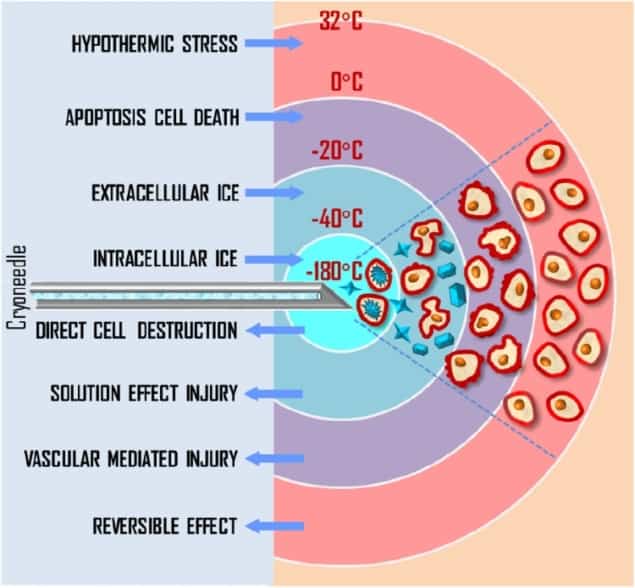
Cryotherapy provides a minimally-invasive technique for the treatment of prostate cancer. The approach, which uses cryoprobes to cool and destroy cancerous tissue, can be performed as an outpatient procedure with rapid recovery, good long-term clinical outcomes and low cost. Despite these benefits, cryotherapy is used far less than surgery and radiotherapy, accounting for just 5% of prostate cancer treatments. This is largely due to inadequate control over temperature, which can lead to either insufficient damage to cancer or unwanted cooling of nearby healthy tissues.
What’s needed is a way to map temperature changes over the entire treated region during cryotherapy. To achieve this, researchers from TomoWave Laboratories and the University of Texas Medical Branch are investigating the use of optoacoustic temperature imaging (OA-Tim). The team has now demonstrated the first in vivo use of OA-Tim during prostate cryotherapy in a canine model (Phys. Med. Biol. 63 064002).
“Adequate control of cryotherapy is critical for both successful destruction of malformations and minimizing damage to adjacent healthy tissues,” said corresponding author Alexander Oraevsky. “With OA-Tim guidance, the treatment accuracy should increase significantly, motivating surgeons to utilize this safe, minimally invasive therapy, rather than quite complex surgery or harmful radiotherapy.”

Improved monitoring
The standard procedure for prostate cancer cryotherapy uses transrectal ultrasound imaging to visualize the prostate and position cryoprobes in the lesion. Pressurized argon gas inside the cryoprobes is cooled to below -100 °C, exposing surrounding tissue to lethal temperatures of below -40 °C.
Currently, this process is monitored using two or three thermal sensors, plus ultrasound imaging to ensure that the expanding iceball does not reach the rectal wall. However, the sparse distribution of sensors within extreme temperature gradients impedes the surgeon’s ability to evaluate tissue damage – and can lead to complications.
Optoacoustic (OA) tomography, which combines pulsed optical excitation and ultrasound detection of the generated pressure waves, offers the potential for real-time temperature mapping of vascularized tissue.
“The OA signal is proportional to temperature, due to the nature of signal generation through thermal expansion of the laser-illuminated volume,” Oraevsky explained. “Thus, OA is a direct method of imaging temperature.”
An in vivo first
Previously, Oraevsky and colleagues demonstrated that OA-Tim can be performed in vascularized tissues. They also established a procedure to convert optoacoustic images to the temperature map, using a calibration equation obtained for blood. In this latest study, they developed a preclinical OA-Tim prototype based on an existing laser OA imaging system, which delivers 805 nm laser pulses, and a modified transrectal ultrasound probe that provides both OA and ultrasound imaging.
The researchers performed in vivo OA-Tim in the longitudinal view during cryotherapy of a canine prostate. The animal was fully anaesthetized during the procedure. Using transrectal ultrasound guidance, they inserted a cryoneedle into the prostate just below the urethra. They also inserted three thermal sensors: Th1, near the cryoprobe to guide the cryotherapy; and Th2 and Th3, located at the rectal wall and Denonvilliers fascia – the most critical areas to avoid during prostate cryotherapy.
Prior to treatment, the researchers obtained an OA reference frame at a known temperature. They then delivered a single freezing event for 3 min 35 s, with the freezing cycle stopped after Th1 showed temperatures below -40°C for 1 min. During cryotherapy, the system recorded OA data at two frames per second, in a 30 x 10 mm region covering the rectum, Denonvilliers fascia and posterior portion of the treated gland.
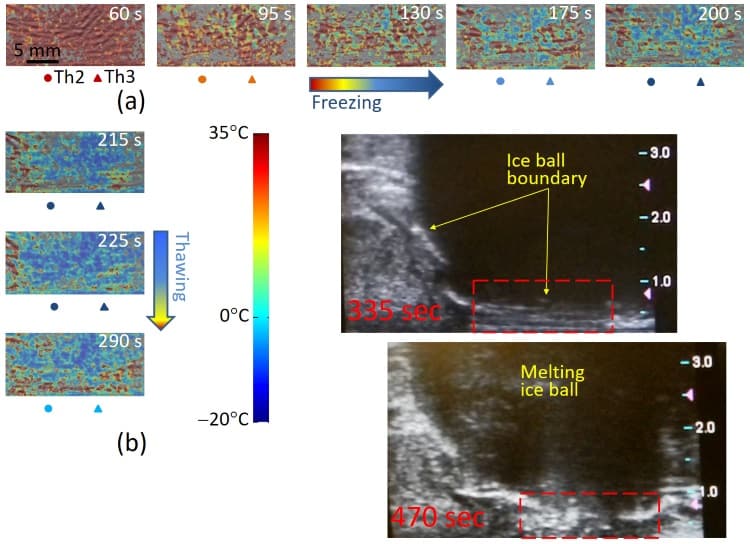
The researchers normalized all OA frames to the reference frame, and used the blood calibration equation to convert each normalized frame to a temperature map. The resultant OA-Tim maps were consistent with the readouts of the invasive thermal sensors, but showed far more detail of the temperature distribution. Since the probes only measure a few specific locations, this non-invasive temperature imaging is far more informative for the surgeon.
To further validate OA-Tim in vivo, the researchers selected a small region-of-interest containing a high-contrast object – a blood vessel or well-perfused tissue area. Temporal profiles from Th2 and Th3 and the profile estimated from the normalized OA intensity showed significant resemblance, except for an overshoot in normalized OA intensity in the first minute of freezing. The temperature was mapped with errors of ±2°C or less, consistent with clinical requirements for monitoring cryotherapy.
“This is the first ever image in a live tissue, so we cannot be too critical of its simplifications,” noted Oraevsky. “The imaged tissue adjacent to the canine prostate was quite homogeneous, in contrast to heterogeneous tissues in a cancerous human prostate. On the other hand, we demonstrated that with a gradual decrease in tissue temperature, our image changed its brightness proportionally. This validates the technological principle.”
The team is now focusing efforts on software development. “It is a challenge to develop software with the capability of temperature imaging at video rates, i.e., in real-time with blood dynamics,” Oraevsky explained. “The speed of calculations is also important for alleviating motion artefacts in live subjects.”



