Blood transplantation is currently the treatment of choice for a wide range of medical conditions, including pathologies such as cancer or acute events like severe burns or trauma. Lack of donated blood is a reason for concern, and when it occurs, can represent the difference between life and death for a patient. For this reason, many researchers are focused on achieving blood production, or haematopoiesis, in vitro.
Naoko Nakamura from Shibaura Institute of Technology and colleagues have described the use of decellularized cancellous bone (DCB) derived from porcine rib as an innovative support for the recruitment of haematopoietic stem cells (the source of all blood cells) in vivo. The team was able to decellularize the cancellous bone fragments without affecting the complex microenvironment required to support the homing of haematopoietic stem cells. Once the cells were recruited in vivo, they began to produce fresh blood cells as they would do in their natural environment (ACS Biomater. Sci. Eng. 10.1021/acsbiomaterials.8b01491).
Description of the haematopoietic niche — the microenvironment in which blood production takes place — is the first milestone in the bold challenge of in vivo blood production. Such a description was produced back in 1973, and since then, researchers have isolated, characterized and expanded the various cell types that can be found in such a niche.
Researchers are now working to understand all the complexities of the haematopoietic microenvironment, in order to reproduce it and to achieve in vitro blood production. Such advancement could be helpful, not only to provide a virtually unlimited source of blood, but also as a valuable tool for the study of blood generation and blood pathologies.
Haematopoietic stem cells have already been isolated and cultured. Moreover, they have also been obtained via the differentiation of induced pluripotent stem cells, making it possible to obtain haematopoietic stem cells without the invasive procedure otherwise needed to isolate them from the patient’s own bone marrow. However, researchers are currently unable to culture them in such a way to obtain the complexity of the native blood. Therefore, efforts are currently underway to produce scaffolds capable of mimicking the native haematopoietic niche.
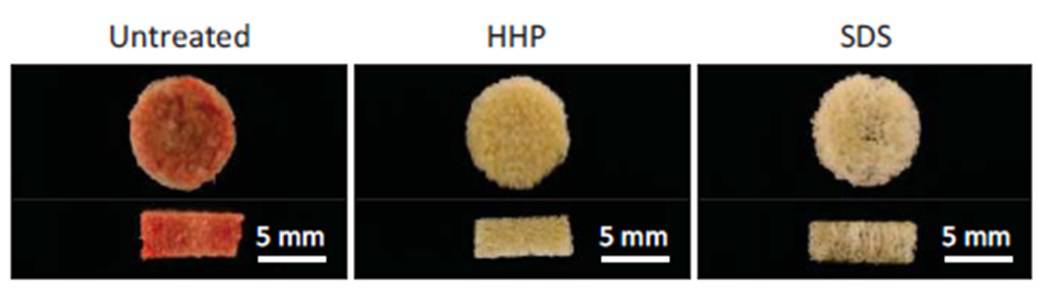
In this study, Nakamura and colleagues evaluated the use of decellularized bone as a scaffold to recruit long-term haematopoietic cells. First, they determined whether high hydrostatic pressure (HHP) or sodium dodecyl sulphate (SDS) treatment would be the best to obtain DCB. To do so, they measured the efficiency in decellularization and evaluated the quality of the obtained scaffolds. The team also examined the ability of DCB to support the growth and differentiation of mesenchymal stem cells, a supporting cell type for the haematopoietic niche that can help recruit haematopoietic stem cells. Although SDS was superior in the removal of DNA residuals, both methods were equally efficient in cell removal and mesenchymal stem cells support, while only HHP preserved an intact extracellular environment.
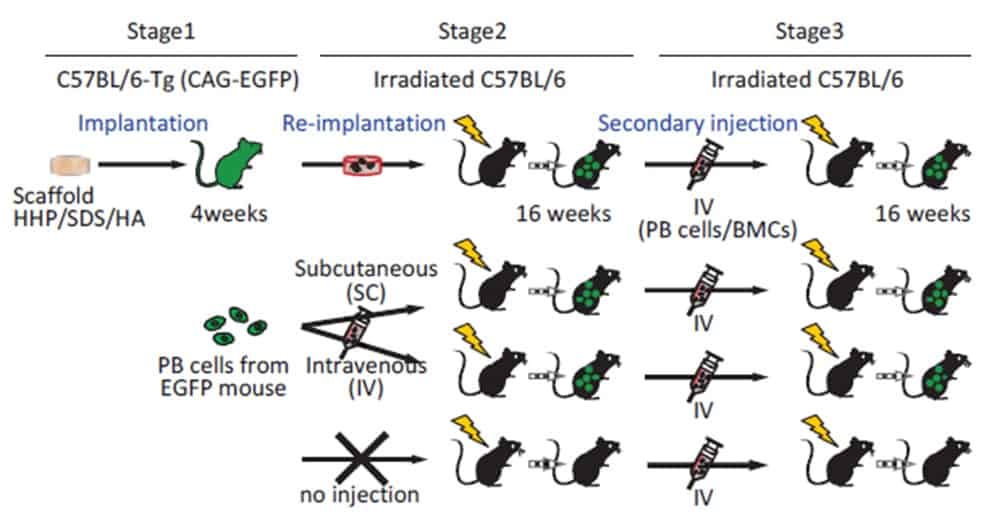
The team subcutaneously implanted the DCBs in mice, and observed that they were appreciably re-cellularized by their host. A closer look at their cellular population four weeks after implantation showed the presence of numerous blood progenitor cells, indicating an active haematopoietic state.
To understand whether such recruited cells were capable of long-term blood production, the researchers further transplanted the scaffolds into a second set of mice. This second group was depleted of its own haematopoietic stem cells through a lethal irradiation at 8 Gy, meaning that these mice couldn’t produce their own blood, and wouldn’t therefore survive long term without bone marrow transplantation. Interestingly, mice that received the in vivo recellularized HHP-DCB scaffolds survived for four weeks after implantation, with newly produced blood cells flowing in their bloodstream.
The results move researchers one step closer to in vitro blood production.