Understanding how the brain responds to a changing environment requires measurement of functional outputs from the whole brain in response to external stimuli. High-resolution PET enables non-invasive imaging of brain function in small animals, and can provide a quantitative 3D map of blood flow, metabolism or receptor-ligand binding throughout the brain. Unfortunately, the need for anaesthesia to avoid motion artefacts not only perturbs many neurological parameters, but also precludes simultaneous brain imaging and behavioural analysis.
Now, a research team from the University of Sydney has developed a technique that enables PET imaging of the brain of an unrestrained rat, while simultaneously recording behavioural outputs following the delivery of stimuli (NeuroImage 10.1016/j.neuroimage.2018.11.051).
To achieve this, the researchers built an open-field PET system based on a commercial preclinical scanner, with a 120 x 200 mm animal enclosure attached to a robotic arm. The robot positions the enclosure in response to the animal’s motion, which is monitored via optical tracking devices at the front and rear of the PET gantry. The team tracked three markers: one attached to the animal’s forehead; one on the moving enclosure; and a reference marker on the gantry.

Lead author Steven Meikle explains that there were three main technical factors that enabled this accomplishment: “Our development of the motion-adaptive observation chamber, which adapts to the animal’s head position within the PET field-of-view; our development of accurate and precise motion tracking of the animal’s head pose during the PET scan; and finally, the adaptation to our system of a motion-compensated list-mode image reconstruction algorithm.”
Receptor binding
Meikle and colleagues first used the technique to estimate changes in binding of the drug raclopride, labelled with 11C, to the dopamine D2 receptor (D2R) in the brains of freely moving rats. They scanned four healthy rats on two consecutive days. For both scans, the rats were given 11C-raclopride (via an indwelling catheter in the right jugular vein) and imaged in the PET system for 60 minutes.
On the first day, 20 minutes after tracer injection, the animals were given unlabelled raclopride at a large enough dose to occupy almost all available D2 receptors and displace the tracer. On the second day, the rats received saline at 20 min instead.
The researchers used the pose information from the motion tracker to perform motion-compensated image reconstruction. PET images integrated over the first and last 20 minutes of the scan (before and after raclopride/saline injection) showed that the 11C-raclopride was indeed displaced by the unlabelled drug, but not by the saline. The estimated onset time of this displacement was 19.5 min on average, in agreement with the time of drug administration. Time-activity curves and displacement curves showed a high degree of inter-subject reproducibility.
The team also measured the average distance travelled by the animals 10 minutes before and after injection of drug or saline. They saw a slight reduction in locomotor activity after injecting unlabelled raclopride, while saline had no effect on behaviour.
Stimulating hyperactivity
Next, the researchers repeated the experiment using the psychostimulant drug amphetamine. While raclopride led to reduced motion, amphetamine stimulates release of dopamine, which competes with 11C-raclopride for the D2R binding sites and is expected to induce robust behavioural changes, such as hyperactivity and stereotypy.
They performed PET scans on three rats on two consecutive days. For each scan, they gave the rats 11C-labelled raclopride and imaged them for 60 minutes. Twenty minutes after tracer injection, they administered either saline (on the first day) or saline plus amphetamine (on the second). Motion-corrected PET images showed an appreciable reduction of the 11C-raclopride D2R binding signal in the striatum after amphetamine administration. The onset of this displacement was consistent with the time of drug administration and peaked 15-20 minutes later.
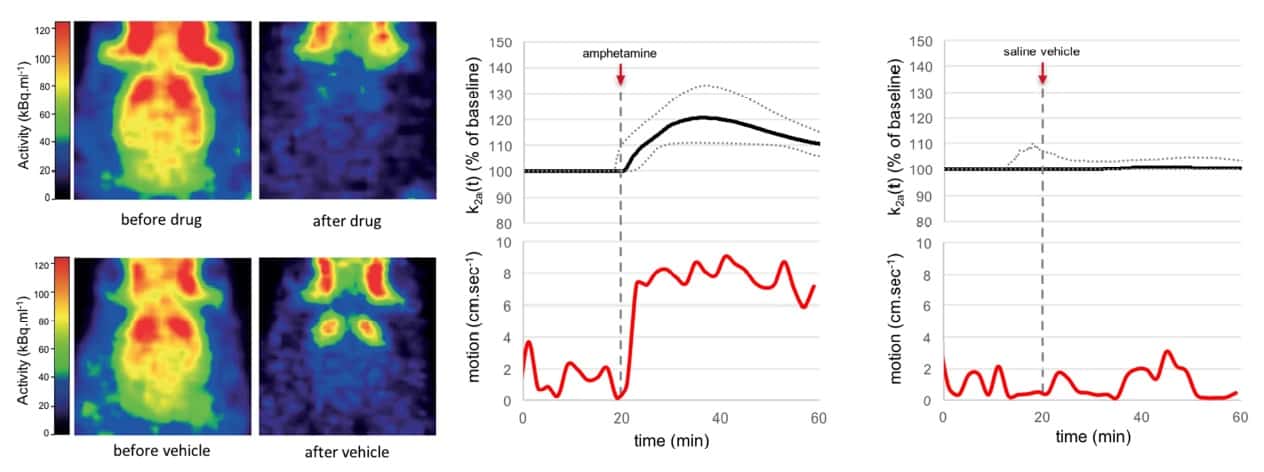
The amphetamine produced a clear increase in locomotor activity shortly after injection, for all three animals, with a pronounced and sustained increase (by 543%) in head motion. Saline did not change the D2R binding or the animal’s behaviour.
For this second experiment, the researchers also observed the rats every minute and scored them according to nine behaviours: sleeping; grooming; locomotion; head-up sniff; head-down sniff; non-specific mouth movements; chewing the chamber; perching on or near the chamber wall; and fast head bobbing.
In a representative animal, behaviour during the first 20 minutes was similar for both scans. After amphetamine administration, the rat showed a marked increase in repetitive locomotive behaviour, repeatedly adopting a perched position and alternately sniffing the base and top of the scanner. In contrast, after saline, the rat maintained moderate to low levels of sniffing in a non-perched position, as well as sleeping and grooming.
The team conclude that their technique can detect and quantify, in awake and freely moving animals, pharmacologically induced displacement of 11C-raclopride from D2 receptors, as well as enabling simultaneous measurement of the animal’s behaviour. These abilities could give rise to important applications in behavioural neuroscience.
“The next stage of this project is to develop a custom-designed high-resolution PET scanner with integrated motion tracking and behavioural response measurements,” Meikle tells Physics World.