MRgRT specialist ViewRay will showcase its MRIdian A3i treatment delivery system at the ASTRO Annual Meeting, with Miami Cancer Institute among the prominent early-adopters

See. Shape. Strike. That’s the shared call-to-arms for a growing international cohort of radiation oncology clinics seeking to transform patient care and treatment outcomes through the deployment of ViewRay’s MRIdian MR-guided radiotherapy (MRgRT) system. Underpinning that collective effort – and key to clinical success – is the MRIdian value proposition. Put simply, diagnostic-quality MR images open up new possibilities for visualization of the tumour target, as well as its surrounding anatomy, with exceptional soft-tissue contrast both prior to and during treatment – allowing clinicians, in turn, to adapt the treatment plan with the patient on the table.
In this way, MRIdian combines online image guidance and adaptive radiotherapy tailored to the unique requirements of each patient – adjusting radiation delivery to address the daily variation in the tumour and surrounding healthy tissue, while enabling the clinician to rework the plan for tumours that respond rapidly to treatment, as well as those that prove unresponsive to standard doses of radiation. Equally significant, the ability to use real-time MR imaging to track the tumour and its environment “on the fly” – automatically turning the beam off if the tumour shifts out of position and on again when it moves back – means it is now routinely possible to increase the radiation dose to diseased tissue in real-time while still protecting adjacent organs-at-risk (OARs) and other critical structures.
The next iteration
As the MRIdian technology push scales to meet demand from existing (and prospective) clinical users, ViewRay will showcase its new MRIdian A3i system at the ASTRO Annual Meeting in San Antonio, Texas, this week. For visitors to the ViewRay booth, the big MRIdian A3i take-aways will likely include streamlining of the on-table adaptive workflow – allowing clinicians, physicists and radiotherapists to collaborate simultaneously and connect remotely during patient treatment. Meanwhile, the real-time, multiplanar, 3D tissue tracking and automated beam-gating functionality mean clinical teams are now able to select up to three different tracking structures (which can include tumour targets and/or OARs) in any combination of coronal, sagittal or axial planes (automatically stopping the beam when any single tracking structure exceeds clinician-defined treatment boundaries).
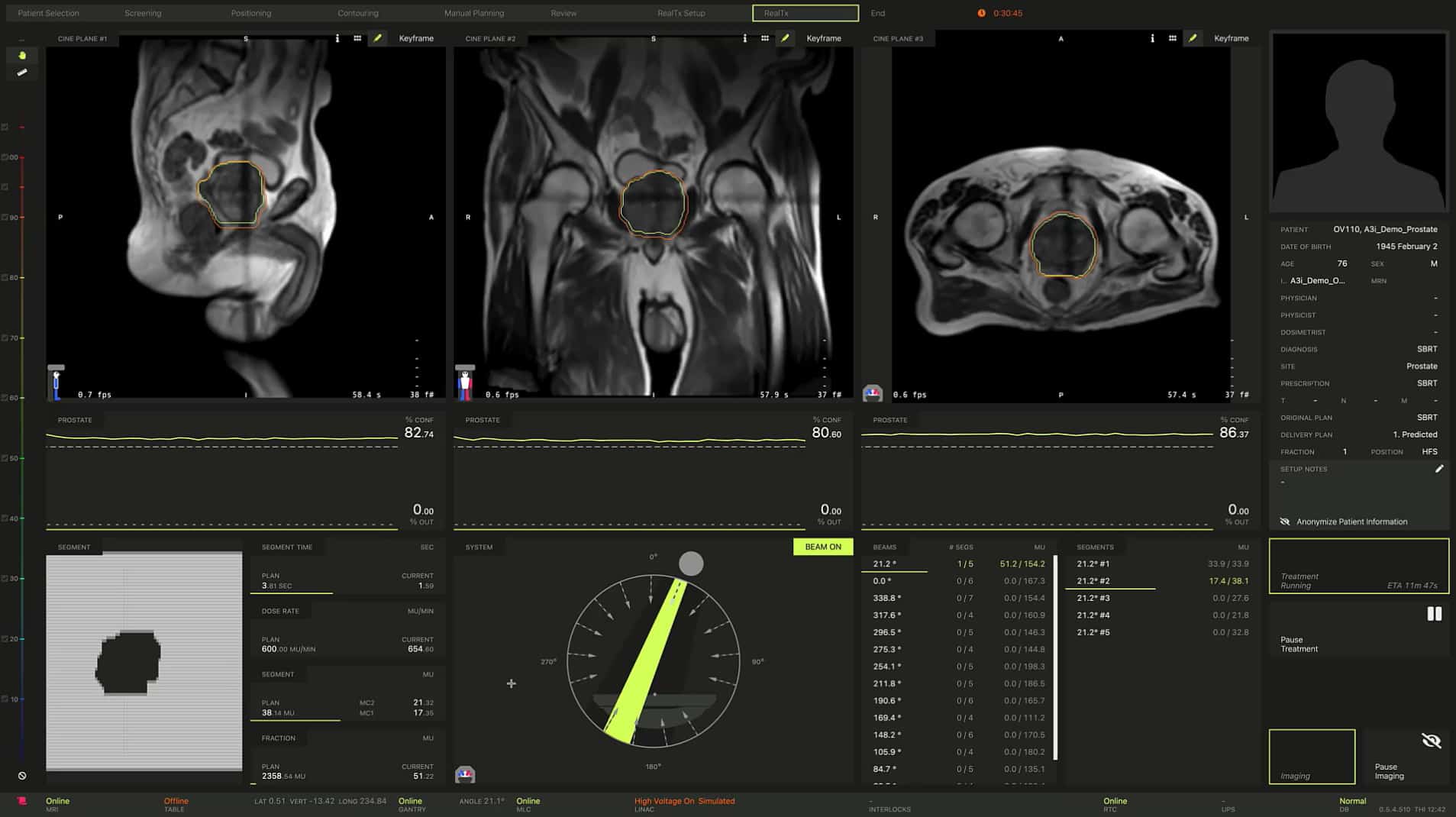
Another key addition within MRIdian A3i is the brain treatment package (BrainTx), comprising a dedicated brain coil and an integrated stereotactic brain immobilization system – the combination of sub-mm volumetric resolution, real-time imaging and automated beam gating extending the utility of MRIdian into cranial stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT).
Enhanced patient experience is also to the fore in ViewRay’s ASTRO narrative, with MRIdian A3i’s integrated patient display allowing the patient to participate, and be in control, during the treatment process. As such, the display provides visual feedback of the real-time imaging to help the patient “hold” the tumour in the correct position for radiation delivery. When the patient needs to relax and take a breath – and the tumour moves out of position – MRIdian’s automated beam gating turns the beam off. Conversely, when the patient holds their breath again, they use the display to keep the tumour in position, with the beam turning back on automatically.
Cutting-edge cancer care
Among the early-adopting clinical users of MRIdian A3i is the Miami Cancer Institute (MCI), part of the Baptist Health South Florida healthcare network. MCI went fully live with MRIdian in 2018 and to date has treated more than 600 cancer patients using ViewRay’s variation on the MRgRT theme. “We describe the radiotherapy programme here as ‘high-tech, high-touch’,” explains Alonso Gutierrez, chief physicist in MCI’s department of radiation oncology. “The ViewRay MR-Linac forms part of an evolving portfolio of cutting-edge technologies that we use to deliver the highest-quality cancer care.”

In large part, the clinical focus of MCI’s MRIdian programme is on stereotactic body radiotherapy (SBRT) and high-dose hypofractionated treatment schemes that exploit the machine’s online MR imaging capability to track moving targets and adapt radiation delivery in real-time. “We’re pushing the boundaries regarding the ablative dose we’re able to deliver to patients safely – using isotoxic dose escalation while respecting the OARs,” notes Gutierrez.
For context, more than 80% of MCI patients treated on the MRIdian system receive five or fewer fractions, with clinical trials underway to evaluate the feasibility of single-fraction treatments for specific disease sites. “What we’ve seen with MRIdian’s motion-management capabilities are clear and sustained benefits regarding accurate and streamlined delivery of dose across a range of disease indications – pancreas, adrenal, liver and lung among them,” he adds.
Listen, learn, deliver
If that’s the back-story, the here-and-now for MCI’s MRIdian programme is all about the roll-out and clinical exploitation of the MRIdian A3i system – a multidisciplinary undertaking that’s headed up by Kathryn Mittauer, a medical physicist and the MRIdian lead at MCI. “Working with ViewRay on the latest iteration of MRIdian has been one of the most rewarding experiences of my career,” she explains. “It’s been invaluable for our MCI team and other institutions to have had direct input on MRIdian A3i development – ultimately improving the functionality of MR/RT for all our collective patients.”
What Mittauer is alluding to is ViewRay’s customer-centric approach to MRgRT innovation and continuous improvement of the MRIdian system. Fundamentally, MRIdian A3i is the result of listening to MRIdian users at the sharp-end of treatment delivery so as to understand – at a granular level – the A to Z of the MRgRT clinical workflow (and the big wins therein). The so-called “Sim Clinics” that Mittauer and colleagues attended at ViewRay’s Mountain View facility in California are a case in point. “These simulated clinics [held over multiple days] provided hands-on experience with the work-in-progress MRIdian A3i system and the opportunity for users to give direct feedback to the product development team,” she explains. “That open dialogue with end-users was central to the MRIdian A3i requirements-gathering process and the prioritization of new features.”

It’s three months since MRIdian A3i was installed and commissioned at MCI and, according to Mittauer, the impact on MRgRT workflow efficiency has been immediate – and impressive. Most notably, MRIdian A3i’s parallel adaptive workflow means the radiation oncology team is able to work collaboratively (and simultaneously) on the patient through localization, contouring and plan review. “As a result, we’re seeing a roughly 30% reduction in overall treatment session time per fraction with MRIdian A3i versus the same workflow for comparable patients before the upgrade,” notes Mittauer.
In terms of the specifics for, say, complex breath-hold SBRT treatments, the MCI team is currently at less than 45 minutes/fraction for the majority of MRgRT patients – from the patient walking into the treatment room and out again – though Mittauer reckons that 30 minutes (or thereabouts) will eventually become the norm thanks to MRIdian A3i. “Greater patient throughput means improved patient experience, with less time spent on the treatment couch,” she explains. “What’s more, the aggregate time savings mean we’ll be able to open up additional MRgRT treatments to more patients while expanding the role of MRgRT to other disease sites.”
Alongside MRIdian A3i workflow efficiencies, the MCI team is also seeing significant upsides from the addition of multiplanar tracking to MRIdian’s MR-guidance capabilities. The goal here: unprecedented, real-time visualization of the tumour and surrounding healthy tissue during treatment, allowing clinicians to safely ablate disease sites where there is significant intra- and/or inter-fraction motion of the target and nearby OARs.
Mittauer cites the example of a multisite, single-isocentre liver patient treated on MCI’s MRIdian system soon after MRIdian A3i went live. “Using a Gd-based contrast agent,” she explains, “we were able to simultaneously track both of the liver lesions in two sagittal planes and safely use a 3 mm treatment margin – something we’d never been able to do before.” The advanced multiplanar, tissue tracking and automated beam gating are also relevant for single-lesion patients, helping the clinical team to see in 3D what’s really going on inside the patient.
“Multiplanar tissue tracking and beam gating offer another level of precision,” adds Mittauer. “Every once in a while, for example, we will have a patient with a lateral displacement that we were unable to see previously because we didn’t have coronal or axial-based tracking. For such cases, we can now make any necessary fine adjustments while the patient is on the table before continuing with treatment.”
The next big thing
From a strategic perspective, Gutierrez is increasingly focusing on what’s next in terms of MCI’s exploitation of the MRIdian A3i system. Near term, he’s excited about the SRS/SRT opportunities afforded by MRIdian A3i, with the radiation oncology team exploring options for related clinical trials. “The new brain treatment package opens up a new application space for MRIdian,” he notes. “We already have a Gamma Knife [from Elekta], a CyberKnife [from Accuray] and are installing other SRS modalities. Having all these stereotactic treatment systems under one roof and being able to compare MRIdian alongside them is going to be really interesting.”
Mittauer, for her part, says the long-run operational priority is to unleash the full potential of MRIdian A3i and, in turn, open up MRgRT treatments to a wider patient population. “Expanding the clinical application of MRgRT is what MRIdian A3i is all about,” she concludes. “It helps, of course, that MCI has a team of clinicians whose default setting is to push the boundaries. As a medical physicist, it’s a privilege to be part of that collective endeavour.”
Adaptive thinking drives MRIdian innovation

As chief scientific officer and founder of ViewRay, James Dempsey is seeking to rewrite the rulebook for radiation oncology and, in so doing, deliver enhanced treatment outcomes at-scale. Here he gives Physics World the headline take on MRIdian A3i’s advanced functionality and the clinical upsides for users of ViewRay’s MRIdian treatment system.
Speed, automation, ease-of-use
One of the priorities for MRgRT users is workflow efficiency, so we have rewritten much of the MRIdian software from the ground up. In MRIdian A3i, for example, we have streamlined the on-table imaging and adaptive radiotherapy algorithms by minimizing manual steps and automating everything we could automate – all of which is reinforced by rapid screen transitions and load times. Those automated workflow steps and contouring tools minimize clinician time and increase patient throughput. New-look treatment planning software is also in the works, which means we’ll be “A3i-ifying” the whole MRIdian software architecture and further streamlining the process of patient care.
Parallel workflows
What we’re calling the remote, parallel, collaborative workflow is a game-changer in MRgRT treatment delivery. MRIdian A3i’s remote portals mean clinical staff can work simultaneously and connect remotely (using video or voice chat) during the patient treatment – in effect, streamlined on-table ART from anywhere. The ability for cross-disciplinary teams to collaborate in this way is liberating and, for many complex adaptive cases, users are telling us that the 20-30 minutes of extra work with a conventional serial workflow is being crushed down to just a few minutes.
Control along multiple coordinates
MRIdian A3i offers real-time, multiplanar tissue tracking and automatic beam gating in up to three planes. Clinicians have the flexibility to select up to three different tracking structures (tumour targets and/or OARs) in any combination of coronal, sagittal or axial planes and to automatically stop the beam when any single structure exceeds the clinician-defined treatment boundaries. It became clear to us, even as early as the simulated clinics in Mountain View, that this capability would open up exciting possibilities for our users, facilitating control of the treatment beam in 3D space rather than just a single plane. Our expectation, over time, is that this additional level of control will deliver improved treatment outcomes.