Proton pencil-beam therapy reduces damage to surrounding tissue compared with conventional radiotherapy, because protons stop at a certain distance within the tissue, whereas photon beams continue through the patient. Therefore, proton beams are used to treat tumours in critical organs of the body, such as the brain.
Particle accelerators deliver a dose of protons at energies sufficient to reach the entire tumour. However, accuracy in proton delivery is limited by uncertainty surrounding the proton beam range.
“Currently we have to irradiate an area with a margin of error around the tumour, to make sure that we actually get the whole tumour,” explains Joost Verburg from Harvard Medical School. “If you are able to precisely measure [protons], you could create a dose distribution that is more focused on the tumour and avoids nearby organs.”
Verburg is part of a team of researchers at Massachusetts General Hospital and Harvard Medical School who have developed a precise proton range detection system for integration into the clinical workflow (Phys. Med. Biol. 63 185019).
Detecting prompt gamma rays
The team’s system detects prompt gamma rays – radiation produced by proton interactions with atomic nuclei within the patient. Roughly 10% of the protons undergo a nuclear reaction that instantaneously emits gamma rays, which escape from the tissue causing no damage to the patient. This provides a real-time, external signal that the researchers wanted to harness for proton range detection. However, Verburg explains, the conditions for detection were “very challenging”.
“In normal gamma ray experiments we get 1000 to maybe 50,000 gamma rays per second, but in this case we get many millions per second. None of the current commercially available gamma ray detection technologies are designed to perform accurate and quantitative measurements under such conditions, so we had to develop a very different type of detection technology.”
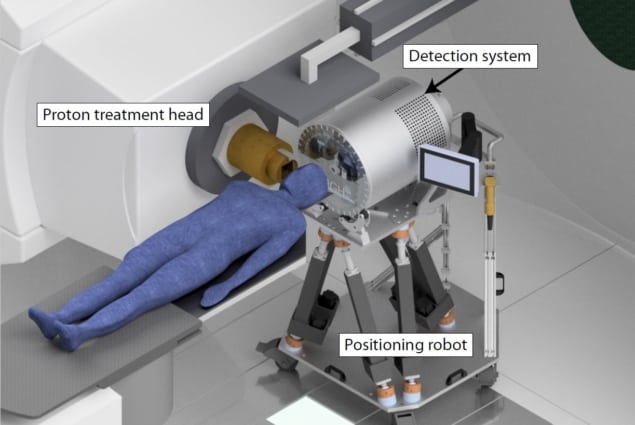
The team customized a scintillation detector, upscaling from their original prototype, to increase the efficiency of gamma ray capture. The gamma rays are converted into visible light, from which the energy and arrival time are calculated. Another important part of the machine, the tungsten collimator, focuses the detector on a specific point on the patient, increasing the proton beam spatial reconstruction accuracy.
The researchers also had to make major developments in detector software, to enable the complex processing of each gamma ray detected and subsequent models to calculate the deposited range and dose of protons.
Modelling nuclear interactions
“We went back to first principles of nuclear physics and tried to model exactly what’s going on in the patient,” says Verburg. “We separately resolved all the different nuclear reactions by looking at the gamma ray energies.” Based on this, the team built a detailed model and used it to predict reaction probabilities from proton energies. This informed Monte Carlo simulations (accelerated on a graphics processing unit) of the absolute range of proton pencil-beams.

Prompt gamma imaging meets error challenges
The detector was tested in the proton beam therapy centre at Massachusetts General Hospital, with different types of plastic blocks used as phantom patient tissue. The detector was able to predict each spot of the distal energy layer to within a mean precision of 1.1 mm, with 95% confidence. This is the first time that this level of accuracy has been achieved under clinically realistic conditions and Verburg describes it as an “exciting” development.
Moving into the clinic
The team is now working on the finishing touches to integrate their detector into the clinical workflow. This includes finalizing the assembly of a positioning robot upon which the detector will be mounted for patient alignment, and conducting a final validation study using an anthropomorphic head phantom in preparation for the upcoming clinical brain tumour trial, expected to begin in late 2018 or early 2019.
“The first time we use this in patients, we’ll just take in vivo measurements and make sure it really works. The next step is to change the treatment plan along the course of the treatment,” says Verburg. To enable clinicians to easily check the treatment plan, and maximize organ sparing, the group will superimpose the calculated proton range and dosage onto CT scans.
“The long-term goal is to use the device to give real-time feedback to the proton therapy system and then fine tune the proton beam in real time,” Verburg tells Physics World.



