Proton CT is proposed as an alternative to X-ray CT for acquiring relative stopping power (RSP) maps for use in proton treatment planning. In parallel, researchers have shown that dual-energy X-ray CT (DECT) improves RSP calculation accuracy over conventional single-energy X-ray CT. A team headed up at LMU Munich, working with partners in the pCT collaboration, has now performed the first direct experimental comparison of the two modalities (Phys. Med. Biol. 10.1088/1361-6560/ab2b72).
“The evolution of DECT is already improving RSP accuracy clinically at some institutions,” says co-senior author Guillaume Landry. “Nevertheless, exploring alternative methods of RSP estimation is important to ensure continued improvement of treatment conformality. As a complete solution to the range uncertainty problem, pCT improves treatment planning and provides low-dose pre-treatment verification that’s not susceptible to metal artefacts. This may make it the preferred solution in the long run.”
The international team compared a phase II preclinical pCT prototype scanner at the Northwestern Medicine Chicago Proton Center with a state-of-the-art diagnostic DECT system. To assess RSP accuracy, they used both systems to image phantoms containing tissue-mimicking inserts of known RSP. They also used Geant4 Monte Carlo code to perform realistic and idealized pCT detector simulations.
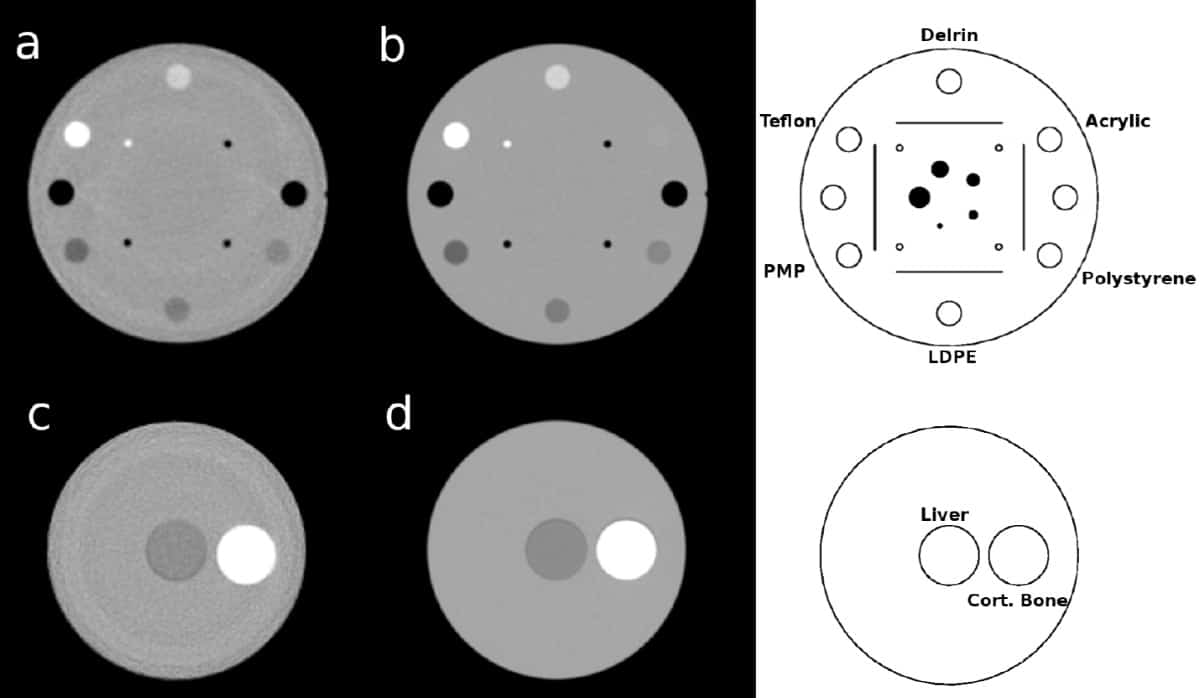
In the experimental pCT scans, the measured RSP accuracy was better than 1% for all inserts except three (polymethylpentene, Delrin and Teflon inserts, with errors of 1.08%, -1.16% and -1.31%, respectively). In DECT scans, only cortical bone and Teflon inserts had errors above 1% (1.17% and 2.38%, respectively). The mean absolute percent error (MAPE) in RSP over all 13 inserts was 0.55% for the prototype pCT scanner and 0.67% for DECT. Excluding the Teflon insert, these values reduced to 0.49% and 0.53%, respectively.
In realistic full-detector pCT simulations, the three central inserts had errors greater than 1%, attributed to simplified modelling of gaps between proton tracker modules in the scanner as air. The MAPE was 0.69%, reducing to 0.50% without the central inserts, thus agreeing with the measurements. In ideal pCT simulations (where the proton’s exact position, direction and energy were scored), the RSP MAPE was 0.17%.
While the researchers did not explore the imaging dose limits of DECT in this study, they expect that pCT will enable volumetric RSP imaging at much lower absorbed (and likely also biologically effective) dose than DECT. “This difference in dose could prove crucial in cases of frequent volumetric isocentric imaging for patient alignment and treatment (re)planning,” notes co-senior author Katia Parodi.
Assessing the artefacts
The experimental and realistically simulated pCT images contained ring artefacts that degraded the RSP MAPE compared with the ideal pCT simulation. In experimental scans, the strongest ring artefacts exceeded 2% in RSP and appeared mostly as RSP overestimation. In the realistic simulations, artefacts reached up to 2% and were mostly RSP underestimation.
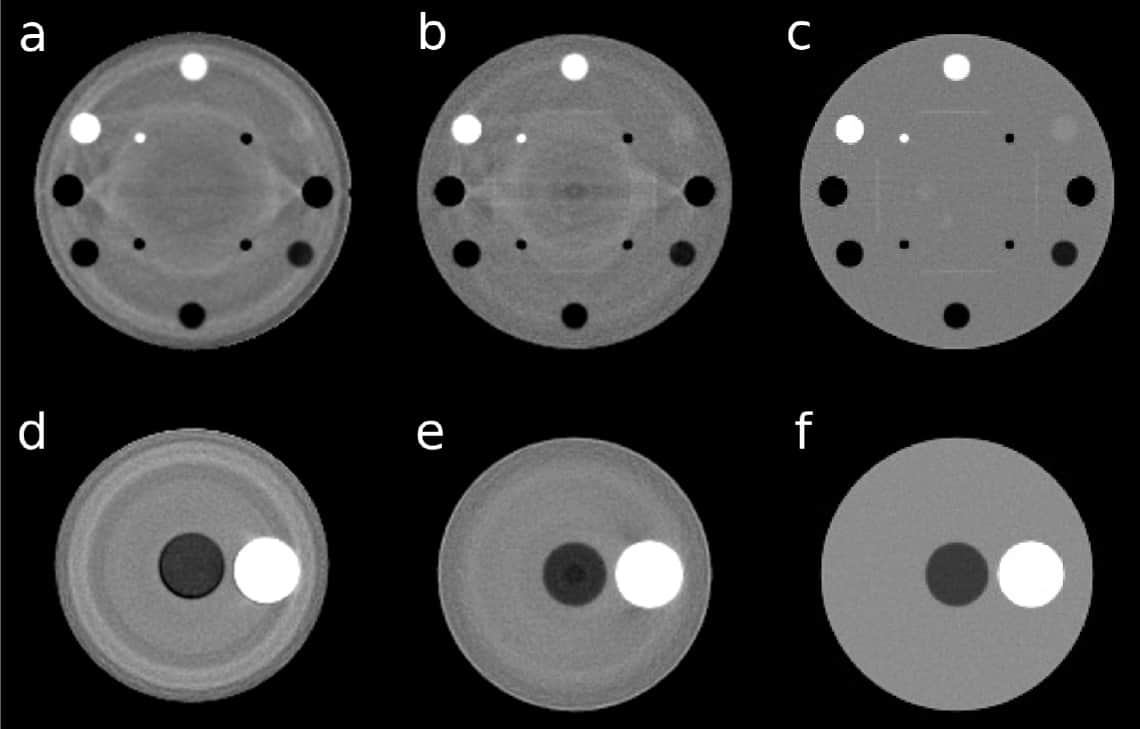
The researchers suggest that ring artefacts appear when protons traverse regions with higher errors in WEPL. These low-accuracy WEPLs arise from inaccuracies in the energy-to-WEPL calibration curve, due to interpolation between different stages of the energy detector, as well as interplay between the energy detector, the beam and the calibration phantom.
“We replaced the original calibration step phantom by a wedge, which reduced the ring artefacts to generally less than 1% of the underlying RSP value,” says co-senior author Reinhard Schulte. “The ideal solution would be to avoid stage interfaces and cover the imaging field with a scanned pencil beam that, depending on knowledge of the phantom thickness from prior imaging data, would adjust the beam energy so that the beam always stops in a single state (energy-modulated proton CT). This solution is currently being explored.”
The realistically simulated pCT images showed a dark (lower RSP) artefact at the centre of a water phantom placed at the isocentre. This artefact, stemming from the exaggerated tracker gap modelling with respect to the experimental setup, was stationary when the water phantom was moved. When the simulation modelled silicon in the tracker gaps instead of air, the dark spot almost disappeared.
The researchers conclude that this direct comparison of RSP accuracy demonstrates the competitive performance of the pCT prototype compared with a state-of-the-art DECT scanner in phantoms. Next, they plan to move on to more realistic tissue samples.
“Our final goal is to push this technology into the clinic, by combining hardware and software improvements of the imaging system with our recent proposal of fluence-modulated imaging, which could open further opportunities for dose reduction,” says lead author George Dedes. “Our ideal simulations suggest that subsequent pCT development cycles have promise to surpass what is currently achievable with latest generation DECT scanners.”