There are many possible therapeutic options for treating patients with cancer. It would be incredibly helpful to be able to predict in advance which of these treatments might be successful. Thanks to research published in the Journal of Nuclear Medicine, we now have a new method to help determine whether a particular tumour might be successfully treated with iron-targeting cancer treatments.
An energetic solution
For cancer cells to endlessly multiply, they need a huge amount of energy. The machinery needed to generate that energy requires a lot of iron as a key building block. We have known for many years that cancer cells are hungry for iron. This has led to therapies being developed that target the significant iron reserves in tumour cells and turn this iron into a weapon to use against the cancerous cells.
Researchers at the University of California, San Francisco, have developed a way to assess the amount of available iron inside a cancer cell and therefore predict whether these new iron-targeting treatments might be effective on a particular patient’s tumour. This parameter has previously been impossible to measure.
“Iron rapidly oxidizes once its cellular environment is disrupted, so the intracellular form can’t be quantified reliably from tumour biopsies,” explains co-senior author Adam Renslo.
Instead, the researchers have developed a radiolabelled molecule that reacts with the available iron in cells and results in it getting stuck in those cells. The amount of this radiotracer retained in a cell is proportional to the amount of iron that was available in the cells initially. The radiolabel is already used widely in PET scans: a safe, detectable, radioactive fluorine-18 atom incorporated into the molecule’s structure. When the radioactive fluorine decays, it emits a positron, which can be detected outside the body using a PET scanner.
While testing this new method, the researchers found that the uptake of their labelled molecule correlated with the amount of an enzyme that processes the iron in the cell – indicating the likelihood of there being more iron present.

Deep-learning model enables rapid lymphoma detection in PET/CT images
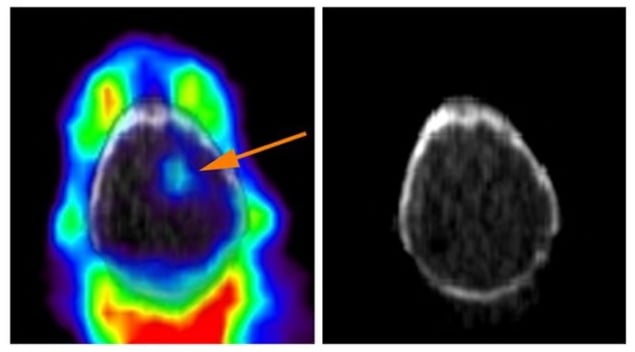
They also found that the success of treating different cancer cell types with iron-targeting drugs could be predicted by the uptake of their labelled molecule. PET images of the brain of a mouse with an implanted tumour clearly revealed the tumour amid the surrounding tissue.
Co-senior author Michael Evans notes that iron dysregulation “occurs in many human disorders, including neurodegenerative and cardiovascular diseases, and inflammation”. He says that being able to use this new tool in patients, to determine how much of this free iron is present and whether treatments targeting it may be successful, represents “an important milestone towards understanding the therapeutic potential” of these treatments.