Medical devices that incorporate hydrogels into silicone rubber could release antibiotics into areas where they are needed most. Erik Brok, Caroline Boudou, Martin Alm and Peter Thomsen describe how neutron scattering is helping researchers to understand and optimize the structure of these silicone-hydrogel networks

Hospital-acquired infections (HAIs) are one of the biggest challenges in modern healthcare. Within this wider problem, urinary-tract infections associated with catheter use are a particular concern: in 2001 a US study found more than half a million cases each year, accounting for approximately 40% of all HAIs (Int. J. Antimicrob. Agents 17 299). Such infections often stem from biofilms that form when a catheter is inserted into a patient’s urethra. These biofilms – which are made up of micro-organisms and their extracellular detritus – act as refuges for bacteria, making infections persistent and difficult to treat. Developing novel catheter materials that resist biofilm attachment is thus a promising strategy for reducing the number of HAIs.
Modified materials
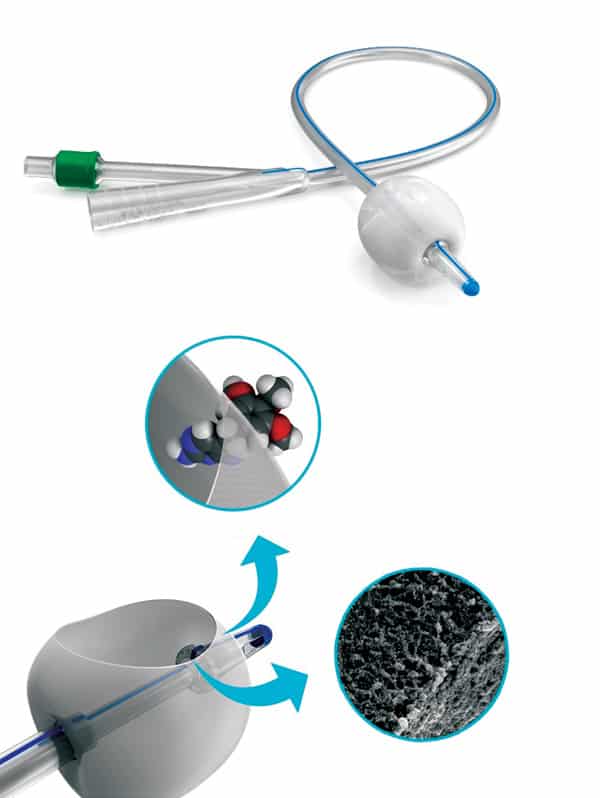
The most common material for urinary catheters, silicone rubber, is a polymer with a backbone consisting of silicon and oxygen. This backbone makes it possible to tailor the material’s texture and elasticity to different applications, and it also makes it highly chemically inert – all excellent characteristics for a medical device. Silicone on its own is, however, hydrophobic, so it is relatively easy for hydrophobic biofilms to adhere to it. This is why BioModics, a Danish medical-supplies company where two of us (MA and PT) are senior managers, has been developing ways of modifying silicone to make it more resistant to biofilm attachment.
At BioModics our approach is to transform silicone by treating it with carbon dioxide at high temperature and pressure. Under these conditions, the carbon dioxide becomes a supercritical fluid – it can penetrate materials like a gas, while also acting as a solvent for the silicone. This process causes the silicone to expand, such that it can be impregnated with hydrophilic molecules that then polymerize inside the silicone network. The result is an interpenetrating polymer network (IPN) of silicone and hydrophilic gel, or “hydrogel”; the hydrophilic nature of this system makes it more difficult for biofilms to adhere to it (Eur. J. Pharm. Biopharm. 94 305).
Making catheters from this hydrophilic hydrogel-silicone could significantly reduce the risk of patients developing catheter-related infections, but BioModics’ patented technology also has potential applications in drug delivery. Whereas silicone on its own repels water, the hydrogel in BioModics’ IPN material serves as a reservoir for liquids in which small hydrophilic molecules – such as antibiotics to destroy any bacteria that do manage to colonize the catheter – could be suspended. This might make it possible to deliver active pharmaceutical ingredients locally instead of systematically, limiting their side effects. Again, the change from a hydrophobic to a hydrophilic material is key.
To improve BioModics’ technology and optimize the material processing conditions for different applications, we wanted to understand precisely how the silicone and hydrogel are distributed through the IPN structure. For example, understanding and controlling properties such as the connectivity and pore sizes of the hydrogel network is critical for ensuring that the network is suited for transporting specific drugs. However, studying the hydrogel structure is tricky because the two polymer networks (silicone and hydrogel) are integrated on the nanoscale, and inspecting them with a light or electron microscope reveals little contrast between the two materials.
To overcome this problem, BioModics turned to a project called LINX, which stands for Linking Industry to Neutrons and X-rays. This initiative was designed to bridge the gap between academic research and industrial R&D in the field of neutron and X-ray scattering, and it facilitates collaborations between three Danish universities (each with its own particular expertise) and companies from a wide range of industries (see Physics World Focus on Neutron Science October 2017 pp17–18). The project’s long-term goal is to help Danish industry make the most of nearby large-scale research facilities such as the European Spallation Source, the MAX IV synchrotron and the European X-Ray Free Electron Laser.
BioModics’ first port of call was the LINX team at the University of Copenhagen, which specializes in small-angle scattering techniques and where one of us (EB) works as a physicist. In both small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS), a beam of radiation (X-rays or neutrons) impinges on a sample and scatters off it. Detectors record the scattered radiation as a function of the scattering angle, which is typically less than 5°. The resulting pattern is related to the Fourier transform of the sample’s structure, so by comparing the scattering data to geometric models, researchers can extract information about the shape and size of the sample’s structure.
Both SAXS and SANS are great for investigating structures at length scales between 1 and 100 nm (and sometimes larger, depending on the specific experimental setup), so they are a good fit for BioModics’ nanoscale silicone–hydrogel IPN. There are, however, some key differences between SAXS and SANS, and these had important consequences for BioModics’ investigations. Whereas X-rays interact with the electrons around the atomic nucleus when they scatter, neutrons interact with the nuclei themselves. This means that neutrons can “see” light elements such as hydrogen, which are practically invisible to X-rays. A subtler but equally important fact is that neutron scattering is isotope-sensitive: if the water in a sample is replaced with heavy water (D2O), the SANS results will look different.
Different tools, different results
Researchers at the University of Copenhagen began by investigating BioModics’ IPN material with SAXS, hoping that these measurements would reveal the hydrogel’s structure. Unfortunately, the electron densities of silicone and hydrogel are nearly the same, so it was not possible to distinguish the two materials using this method. In fact, the observed X-ray scattering profile turned out to be dominated by scattering from the silica (SiO2) particles used as filler to make the silicone more mechanically stable.
After this setback, the team turned to SANS, for which the contrast between silicone and hydrogel is better, especially if the hydrogel is loaded with D2O. Using SANS did, however, present some initial hurdles. While SAXS experiments can be performed using a conventional X-ray tube available in many laboratories, SANS requires a nuclear reactor or a spallation source based on a particle accelerator. These large-scale experimental facilities are usually reserved for academic research, and although industry-access programmes exist, the usual rate for proprietary beam time is thousands of euros per day.
To overcome this financial barrier, BioModics applied for beam time via a project called SINE2020, which offers short periods of free beam time to companies that want to find out whether neutron scattering is a feasible technique for studying their materials. BioModics was awarded beam time at the Institut Laue-Langevin (ILL), a major neutron-scattering facility in Grenoble, France, where one of us (CB) works as an industrial liaison officer. After BioModics shipped their materials from Denmark to France, scientists at the ILL performed measurements on samples of pure silicone, dry IPN and on IPN samples that had been soaked in D2O for a week.
Making catheters from hydrophilic hydrogel-silicone could significantly
reduce the risk of catheter-related infections
The SANS data from pure silicone and dry IPN were strikingly similar, which unfortunately means that the strongest scattering signal was still coming from the filler material. However, SANS data on the samples soaked in D2O looked quite different. This made it possible to differentiate the signal of the hydrogel from that of the silicone, and thus to learn about the IPN structure. By applying fractal network models to the data, we succeeded in deriving a characteristic correlation length related to the pore size of the hydrogel, as well as a parameter describing the roughness of the interfaces between hydrogel and silicone. After doing this for two samples with a different silicone base but the same amount of hydrogel, we found that the samples had different correlation lengths (58.6 nm and 32.5 nm), while the interface between silicone and hydrogel was also markedly different. In one sample the interface was quite smooth, whereas in the other it was rough or jagged.
Drug delivery
This result suggests that an IPN made from the material with the smoother interface may be the better of the two for transporting drug molecules. Although this finding would, of course, need to be confirmed by more direct measurements of drug transport through the material, it was intriguing enough for BioModics to purchase additional beam time at the FRM-II research reactor in Garching, Germany. In a later series of experiments, we mapped out how the IPN’s structure changed as a function of hydrogel content, in order to determine how to produce a structure that is optimized for drug delivery. These data, taken in summer 2017, are being analysed by researchers in the LINX team at Copenhagen, but so far the results are promising. Ultimately, we expect that the project will help BioModics select the optimal silicone type, hydrogel content, and possibly other parameters, in order to achieve the best possible properties for our devices – including an optimal hydrogel structure for drug delivery and optimal mechanical properties, such as softness and flexibility, for the catheter material. In the fight against catheter-related infections, these are useful weapons.



