It is well-known that a radioactive substance follows a fixed exponential decay, no matter what you do to it. The fact has been set in stone since 1930 when the “father” of nuclear physics Ernest Rutherford, together with James Chadwick and Charles Ellis, concluded in their definitive Radiations from Radioactive Substances that “the rate of transformation…is a constant under all conditions.”
But this is no longer the view of a pair of physicists in the US. Ephraim Fischbach and Jere Jenkins of Purdue University in Indiana are claiming that, far from being fixed, certain decay “constants” are influenced by the Sun. It is a claim that is drawing mixed reactions from others in the physics community, not least because it implies that decades of established science is flawed.
It’s a gigantic effect…It sounds as though it’s related to solar activity, but it really can’t be John Barrow, Cambridge University
Annual modulation
Fischbach and Jenkins first began looking for fluctuations in nuclear decays in 2006 after they came across the report of an experiment performed at Brookhaven National Laboratory (BNL), New York, between 1982 and 1986. The BNL team found that over that period the decay constant of silicon–32 — relative to a long-lived standard — modulated around its usual value of about 172 years by the order of 0.1%. What is more, the modulation appeared to be almost in phase with the varying distance of the Earth to the Sun: in January, when the Earth is closest, the decay rate was faster; in July, when the Earth is farthest, it was slower.
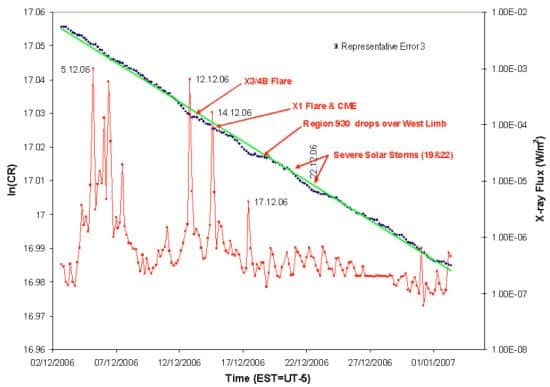
The Purdue researchers were intrigued by the modulation in the BNL data, and in late 2006 began monitoring another nuclear isotope, manganese–54, for unexpected fluctuations. Initially the manganese’s decay seemed to closely follow the usual exponential law. But on 13 December Jenkins caught a story by chance on FOX News about an unusually large solar flare, prompting him and Fischbach to compare their manganese data with X-ray readings from satellites.
They discovered that a spike in X-ray flux associated with the flare roughly coincided with a dip in the manganese’s decay rate. Two days later, an X-ray spike from a second solar flare coincided with another, though very faint, dip. Then, on 17 December, a third X-ray spike accompanied yet another dip, which was more prominent (see above figure).
The Purdue researchers submitted a paper on the solar flare correlations to Physical Review Letters but it was rejected, they say, because there was no mechanism to back it up (they have since uploaded the preprint to arXiv:0808.3156). Undeterred, they began searching the literature for other records of fluctuating decay rates, and indeed this year they found another example in a 15 year-long experiment completed in 1998 at the Physikalisch–Technische Bundesanstalt (PTB) in Germany. As in the BNL experiment, the PTB experiment showed an annual modulation in a decay constant, though this time for the nuclear isotope radium–226. “We were hoping that the identification of fluctuations in other data would lend support to the idea that solar activity could influence decay rates,” explains Fischbach.
What we are showing is that decay constants are apparently not fundamental constants of nature Ephraim Fischbach, Purdue University
New physics?
Fischbach — who made a name for himself in the late 1980s by claiming the presence of a “fifth force” in addition to the four fundamental forces of nature — says he and Jenkins have had “enormous interest” from international colleagues who have jumped at the possibility of new physics. Yet many are not convinced there is anything to be excited about. “It is often difficult for an outsider — i.e. someone who has not built and run an experiment — to consider all possible systematic effects,” says Michael Smy of the University of California, Irvine. “Long-range time variations are notoriously difficult measurements vulnerable to systematic effects.”
Even if the decay-rate correlations with solar flares and the Earth–Sun distance are more than a coincidence, they raise the question of precisely what solar activity is causing the effect. In a more recent paper submitted to Physical Review Letters (preprint at arXiv:0808.3283), the Purdue researchers suggest that the radioactive nuclei are somehow affected by solar neutrinos.
The trouble with this interpretation is that neutrinos are only susceptible to the weak interaction, which governs beta decay. Although the silicon in the BNL experiment beta decays, the radium in the PTB experiment alpha decays — a process that is governed by the strong interaction. Nonetheless, Fischbach and Jenkins think radium exhibits the modulation because many of its decay products — such as lead–214 and bismuth–214 — do in fact beta-decay.
The Purdue researchers use this reasoning to explain why Peter Cooper, a physicist from Fermilab, Illinois, found no decay-rate fluctuation in an extraterrestrial version of the BNL and PTB experiments in a preprint uploaded last week (arXiv:0809.4248). Taking data from NASA’s Cassini probe, Cooper noted that the decay of the plutonium–238 thermoelectric generators on board scarcely veered from the usual exponential law as the spacecraft went as close to the Sun as Venus and as far as Saturn. But Fischbach and Jenkins point out that, considering the plutonium–238 decay chain, it would take a very long time for Cassini’s generators to build up any isotopes that beta-decay.
Long-range time variations are notoriously difficult measurements vulnerable to systematic effects Michael Smy, University of California in Irvine
‘Wild explanations’
As more scientists hear about the claimed relation of decay constants to solar activity, reactions vary from disbelief to rejection. “I don’t understand this at all, what it could possibly be,” says John Barrow of Cambridge University. “It’s a gigantic effect…It sounds as though it’s related [to solar activity], but it really can’t be.”
“If a student brought this to me I would advise them to understand their experiment before they start coming up with wild correlations of detector noise with natural phenomena picked at random,” says David Wark of Imperial College London. “You have to prove that something needs to be explained before you come up with wild explanations.”
Meanwhile, the Purdue researchers have just found yet another example of the decay-rate annual modulation — this time by a US paediatrician who was investigating the decay of plutonium–238–beryllium in 1990. “What our data are showing is that the half lives, or the decay constants, are apparently not fundamental constants of nature, but appear to be affected by solar activity,” says Fischbach. “To summarize, what we are showing is that the decay constant is not really a constant.”