Lying between the microwave and infrared regions of the electromagnetic spectrum, terahertz (THz) radiation offers great promise for medical and biological applications. The THz band – frequencies of 0.3-3 × 1012 Hz – provides a unique view into the interior of biological cells and offers a non-ionizing modality for imaging cancer. And with the introduction of laboratory THz sources and sensitive detectors, could we soon see THz technologies make significant impact in the clinic?

“We’re living at a time when the THz region of the spectrum is now becoming accessible,” said Peter Weightman from the University of Liverpool. “It’s a new tool that we hope may give us advances in cancer management.”
Weightman was a speaker at the recent meeting “Towards the THz Imaging of Cancer“. The event brought together researchers, clinicians and industry players to explore how to transition THz imaging into an effective clinical tool.
Studying single cells
The first speaker, Norbert Klein from Imperial College London, discussed single cell detection based on markers such as cell size or water content, which can be measured using physical techniques. Measurements at THz and microwave frequencies, for example, are sensitive to the water content of a cell, enabling rapid cell characterization without requiring labelling.
“The uniqueness of the microwave to THz range is that it can look inside the cell, but is not yet limited by scattering,” Klein explained. “That’s why this window is of special interest. This is a new form of cancer cell diagnostics that may be complementary to other approaches.”

Klein presented evidence for correlation between microwave response and cancer cell aggressiveness. “We don’t yet know how much we can access with THz, we may see better at THz than microwave frequencies, but this is all early work and more studies are needed,” he added.
Klein and his team developed a cavity-coupled resonator system for fast measurement of flowing cells at 10 GHz (sub-THz). They combined a split-ring resonator with a dielectric resonator, and integrated this into a microfluidic chip. He pointed out that the device is simple to make, low in cost and, in principle, could be extended to 100 GHz (0.1 THz).
They used the device to detect flowing polystyrene microspheres and perform the first microwave measurements of flowing (mouse myoblast) cells. The signals were dependent upon cell volume, providing a fast and accurate way to measure cell size distribution. Potential applications include examining blood samples for circulating tumour cells, which are larger than white blood cells.
For THz detection of single cells, Klein described the use of a silicon photonic crystal resonator to measure blood cell suspensions. “Water content measurement in a single cell is possible,” he concluded. “We have demonstrated this at microwave frequencies and believe it is possible at THz.”
Klein noted that a lab-on-a-chip system that combines rapid measurements of cell size and water content could be a game changer for cancer diagnostics.
In vivo options
Emma Pickwell-MacPherson from the University of Warwick, examined the challenges of in vivo THz imaging. “We have seen an increase in THz imaging in vivo recently,” she said.
Examples include screening of diabetic foot syndrome, where THz imaging reveals differences in tissue water content between diabetics and controls; monitoring scar healing, in which THz can image subtle tissue changes when visible changes are no longer apparent; and even non-contact THz imaging of the corneal surface.
THz techniques can also detect differences between normal and cancerous tissues. In vivo measurement, however, is challenging, due to the substantial number of variables that need to be controlled. “Measuring the same region of skin each time for comparative studies is tricky,” Pickwell-MacPherson explained. “Skin structure changes with position, pressure, time of day, moisturizer and so on, we need a repeatable protocol.”
In vivo skin measurements are usually performed in reflectance mode, by placing the skin on a quartz window. This, however, occludes the skin, changing its water content and thus decreasing the amplitude of the THz signal, particularly in the first five minutes. “We need to know how to model this effect and compensate for it,” explained Pickwell-MacPherson.
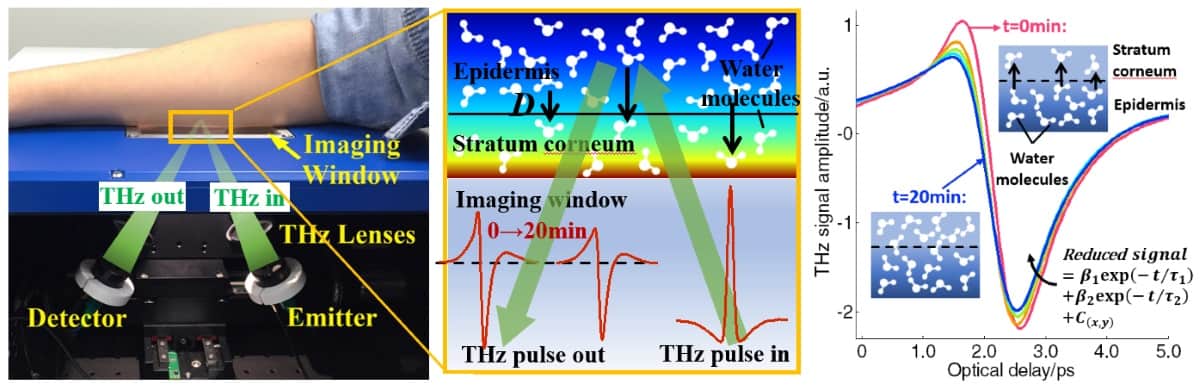
Another consideration is variation in the pressure between the skin and the window, which can also affect measurements and required compensation. Pickwell-MacPherson’s team has studied the use of a pressure sensor to monitor such changes.
THz measurements are affected by both the skin’s thickness and its refractive index (hydration). However, the skin hydration also affects its thickness, as skin tends to expand slightly as it hydrates, complicating the analysis of measured signals. One alternative approach is to use ellipsometry, which measures the refractive index independently of thickness.
With this aim, Pickwell-MacPherson and her team have constructed a THz ellipsometer. They noted that the ellipsometry results had much smaller error bars at high frequencies, overcoming some of the difficulties of opaque sample characterization. “Ellipsometry may be ready for in vivo studies soon,” she concluded.
The industry take
Phil Taday of TeraView described the emergence and development of commercial THz instrumentation. TeraView was spun out of Toshiba Research Labs at Cambridge University in 2001, at which time THz systems were the size of optical tables. In 2003, the company launched a smaller, trolley-mounted THz pulsed spectroscopy system, which could differentiate basal cell carcinoma (BCC) from normal tissue samples via absorption and refractive index measurements.
The next development enabled THz imaging, using reflectance mode and a quartz plate. This system could visualize tumour margins, for example to guide surgical excision of skin cancers and ensure all cancer cells are removed.
TeraView has also developed a handheld fibre-coupled THz imaging probe to view excision margins in breast cancer surgery. Here, the THz response could differentiate fat, fibrous tissue and tumour with a sensitivity of 82% and a specificity of more than 92%. A trial of this approach is ongoing at Guy’s Hospital in London.
The company’s latest device is the TeraPulse 4000, which can perform THz imaging and spectroscopy in both transmission and reflection modes. “This is more mobile, we are getting to the stage where the system is now desktop sized,” said Taday.
One future incarnation could be a device for endoscopic THz imaging, for monitoring Barrett’s oesophagus for signs of cancer, for example. Taday said that this should be feasible, noting that THz emitters could be miniaturized to fit into an endoscope channel, but that shrinking the silicon optics used to control the THz beam may perhaps be harder.
High power
In the final presentation, Weightman looked at how THz characterization of tissue could be exploited for cancer diagnosis. He emphasized the need for intense THz sources to perform experiments. “We know that water is very important for biological systems, so if we want to understand the function of DNA in the THz region we have to work in water,” he explained. “The problem is that 1 mm of water attenuates THz by a factor of 1018.”
Current laboratory sources produce THz radiation with intensities of about 100 µW to 10 mW. Energy-recovery linacs, however, generate a peak power of 10 kW, while free electron lasers can generate MW. Such powerful sources allow investigations into how THz radiation impacts the activity, damage and repair of DNA, and whether it changes the expression of genes.
One important question is whether THz radiation is safe. Weightman described a series of experiments performed at the ALICE energy-recovery linac at Daresbury Laboratory, which had a tissue culture facility for THz irradiation of cells within an incubator. “We found that THz radiation had no effect on the morphology, attachment, proliferation or differentiation of several types of cells,” he said.
Weightman noted, however, that another group has shown that intense THz radiation changed the activity of genes in skin cells. He suggested that the live cells used in his studies may have been damaged but then repaired themselves. More experiments are required, and are planned.
With many unanswered questions, Weightman believes it’s essential to perform basic science studies before transitioning to the clinic. “Clinicians want something that they can use in the operating theatre, but at the moment we don’t know what we can tell them,” he explained.
Looking ahead
In panel discussions, the speakers considered the future potential of THz imaging. Pickwell-MacPherson suggested that achieving in vivo THz imaging will require updated instrumentation, pressure sensors and non-contact measurement systems. Klein suggested that it may be optimal to combine infrared, THz and microwave imaging into one device.
“We are still in the process of understanding what THz can tell us,” said Klein. “But once this is found, maybe progression to clinical trials can be quick. If water content and cell size are important, we could be ready in quite a short time for pilot clinical studies. I would encourage to bring clinicians, technologists and physicists to one table.”
Taday emphasized the importance of miniaturization. “That’s down to people like us to drive forward,” he said. There’s also a need to work with academics on areas such as signal processing and how to extract the most from these signals. He added that faster ways to scan the beam are needed, and that array detectors, or THz CCDs, are another key development.
For THz techniques to appeal to the healthcare sector, they need to compete with existing technologies on a real problem, such as cancer management, for example, where anything that could detect early disease is enormously important. Another possibility is rapid assessment of lymph nodes during surgery. And perhaps THz could even be used therapeutically, to preferentially heat and destroy cancer cells.
Weightman pointed out that distinguishing cancer from non-cancer is not difficult, histology can do that. What’s more challenging is to determine whether a tissue will turn cancerous, whether a lesion will progress. “Once you know the target, then you can develop the instruments,” he said.
- Participants from the day are taking forward a THz network, to encourage clinicians and THz physicists to develop a coherent strategy for THz-based medical instrumentation and diagnostics. Rob Donnan (r.donnan@qmul.ac.uk) is the point-of-contact for readers interested in joining the network.



