“It’s not just about navigating the leap from the lab to market, but also from the market back into the lab,” said Anita Mahadevan-Jansen, at the recent Photonics West BiOS conference.
Presenting in the BiOS Hot Topics session, Mahadevan-Jansen explained that, in addition to the various essential stages required to translate a new medical technique from the laboratory into the clinic, feedback in the other direction is also invaluable to optimize a newly launched technology for patient care. She illustrated this idea with an application developed by her research group at Vanderbilt University – the use of optical spectroscopy to guide endocrine surgery.
One of biggest challenges during surgery of the endocrine glands (usually the thyroid or parathyroid glands) is protecting the parathyroid gland, which is the only organ in body that regulates calcium. The post-operative rate of hypoparathyroidism, which causes blood calcium levels to fall and blood phosphorus levels to rise, can be up to 60%. This problem arose because the only way for a surgeon to find, and avoid, parathyroid glands was by visual inspection.
“In our lab, we discovered that the parathyroid glands have a really strong autofluorescence in the near-infrared,” said Mahadevan-Jansen. The team showed that parathyroid tissue exhibited up to 10 times greater fluorescence signal than other tissues in the neck. In a study of 137 patients having thyroid or parathyroid surgery, detecting this autofluorescence identified both normal and diseased parathyroid glands, in real time, with 97% accuracy.
Mahadevan-Jansen described the device’s progress from the initial discovery in 2008 to a clinical system that was FDA approved in 2018 and CE marked earlier this year. “This was a long journey considering that it is simply based on autofluorescence, no dyes, and the technology is fairly straightforward, just looking at the ratio of signal between two tissue types,” she said.

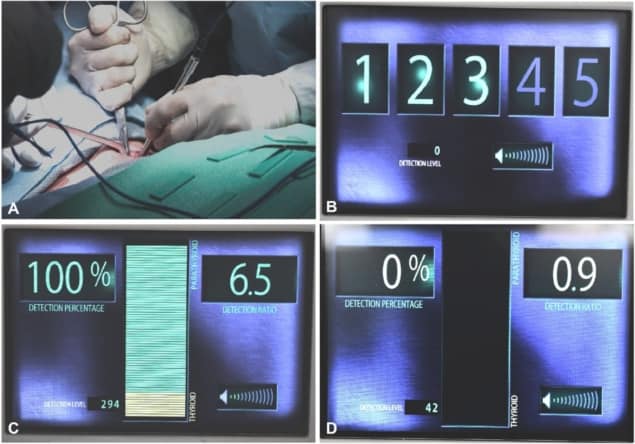
In collaboration with Ai Biomed, the team developed a probe-based clinical system called the PTeye. The device is used intra-operatively, and emits a sound when the fibre-optic probe is near parathyroid tissue. “Yet we came to realise that just because a technology is approved and launched into the surgical community, this does not mean our job is done. The lessons we’re learning since its launch have been tremendous,” Mahadevan-Jansen noted.
The researchers had demonstrated that the autofluorescence technique could find parathyroid glands, but once the device began being used in a clinical setting, they needed to understand how would affect patient care, as well as how surgeons are using the new technology. As such, the PTeye is now being studied in two ongoing clinical trials at four sites, with surgeons using it to guide their surgical procedures in both thyroidectomy and parathyroidectomy cases.
Early results from the trials demonstrate that the technology has an accuracy of 94.3% for identifying parathyroid tissue, with a positive predictive value of 93% and a negative predictive value of 100%. The team saw that the device was definitely helpful, even for an experienced surgeon, particularly in long and complex cases. And for a less experienced surgeon, PTeye had a significant impact, both in building confidence and increasing their efficiency.
“It’s still too early to see the long-term post-operative hypoparathyroidism rate, but I hope to have that over the next year as we continue to accrue patients,” said Mahadevan-Jansen.
And as more surgeons began to use this new device, the team started to see other anecdotal issues arise. For example, the system requires five initial measurements on the thyroid to establish a baseline; but if this baseline is defined improperly, this can result in false positives. Establishing and sharing a procedure to achieve accurate baselining is essential. Another finding is that brown fat, often seen in younger patients, has bright autofluorescence in a similar spectral region.
The team now needs to tackle these issues and understand their impact on the use of the device. Other ongoing challenges include addressing the cost associated with single-use probes, determining whether imaging or spectroscopy is preferable and investigating the identity of the fluorophore – while parathyroid tissue exhibits bright autofluorescence at 822 nm, it’s not clear exactly what this emission is due to.
“We have developed near-infrared autofluorescence as a clinical guidance tool for neck surgery, to confirm the parathyroid,” Mahadevan-Jansen concluded. “This technology is now available commercially, both as a fibre-optic probe-based device and an imaging device. Acceptance by clinicians is still in progress but we are starting to see surgeons really excited about using this.”



