Advances in positron emission tomography are providing detailed images of biological processes in small animals that promise breakthroughs in molecular medicine.
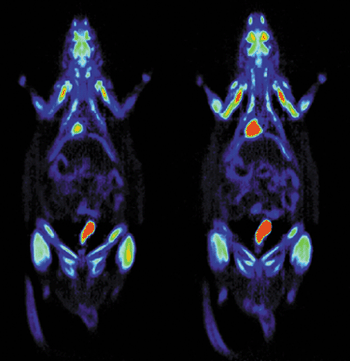
The sequencing of the human genome, along with other advances in the fields of molecular biology, genomics and proteomics, is leading to the discovery of large numbers of genes and proteins. Many of these are implicated in human disease and are therefore possible targets for drug development. We are all eagerly awaiting the new era of molecular medicine, when drugs are precisely tailored to our specific genetic or molecular profile, and when diseases such as cancer and Alzheimer’s can be treated easily without side effects.
While biologists are generating a wealth of information about many diseases, the translation of this knowledge from the test tube to the clinic has been slow and challenging. This is exemplified by the tragic and mystifying death of teenager Jesse Gelsinger, a participant in a gene-therapy trial at the University of Pennsylvania, and the limited success of many similar clinical trials around the world.
To be more efficient at converting new knowledge into effective treatments, we need to be able to answer a number of important questions. Which of the many genes and proteins that may be implicated in a particular disease should we target with new drugs? And once the target is chosen, there are enormous numbers of substances that could interact with or alter those targets. How do we sift through these many possibilities and determine which ones will work as drugs? How do we know if and when the results obtained in cell cultures, tissue preparations and animal models can be extrapolated to humans?
Successful clinical trials require large numbers of patients, so the number of new drugs that can be tested in humans is very limited. The process is also very expensive. We therefore need to make sure that only those drugs with a very high chance of success reach clinical trials. We also know that different patients respond differently to the same drug. So how do we select those patients who have the appropriate genetic or molecular profile to benefit from our new drug? And finally, how do we monitor the drug treatment to ensure at an early stage that the drug is going where it is supposed to go, and doing what it is supposed to do?
The answers to these challenging questions will require new scientific breakthroughs involving many different technologies. One set of technologies that looks poised to play a role is non-invasive imaging techniques that enable molecular targets and genetic processes to be visualized and measured in living subjects.
This is the emerging field of molecular imaging. Physicists, engineers and chemists are playing a crucial role by developing a whole new generation of imaging systems and contrast agents. They include magnetic resonance imaging with paramagnetic atoms, optical-imaging approaches using fluorescence or bioluminescent molecules, and techniques based on radioactive tracers, such as positron emission tomography (PET). These non-invasive techniques allow us to look inside the body, and they provide a window on biology in action.
Principles of PET imaging
In PET, tiny amounts of a biologically interesting molecule are labelled with a radionuclide that decays by emitting a positron. Most of the short-lived radionuclides that are suitable for imaging are isotopes of biologically ubiquitous elements and can be produced in compact biomedical cyclotrons (see table). This means that biomolecules can be labelled by direct isotopic substitution – for example a carbon-12 atom can be replaced with a radioactive carbon-11 atom, which has exactly the same biochemical properties and a half-life of 20 minutes.
One common radiotracer is 18F-fluorodeoxyglucose (FDG), an analogue of glucose that is labelled with the positron-emitting isotope fluorine-18 and is injected into a vein. Radiotracers like FDG are designed so that they spread throughout the body but only accumulate in tissues when they encounter a specific enzyme, protein or gene. The radiotracer builds up by binding to proteins on the cell surface, known as receptors, or by becoming trapped in the cells, as metabolic or enzymatic processes alter its chemical structure.
The distribution of the radiotracer therefore provides information on the distribution and concentration of specific molecular targets in the body, such as receptors, enzymes and transporters. In addition, how quickly the radiotracer accumulates in or clears from tissues often relates to the rates of biological processes in the body (for example rates of transport, synthesis, metabolism and excretion).
However, the radiotracer can also be a drug that is labelled with a positron-emitting radionuclide, in which case its distribution as a function of time mimics that of the drug. Exactly what is measured in a particular study depends on the radiotracer used. The goal is to design radiotracers that are specific to particular molecular targets or genetic processes, and there are many examples of PET radiotracers that meet this challenge.
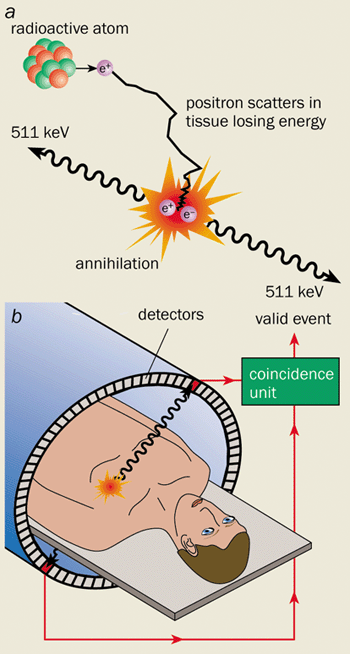
The radiotracer’s distribution in the body is determined by measuring the radioactive decay products with a PET scanner. When a nucleus decays by positron emission, a proton in the nucleus converts into a neutron, and a positron and a neutrino are ejected. The neutrino leaves the scene without a trace, while the positron rapidly annihilates with a nearby electron.
Annihilation results in the mass of the two particles being converted into energy. Since the electron and positron are essentially at rest at the time of annihilation, this energy is released in a very specific manner, namely as the simultaneous emission of two photons almost 180° apart, each with 511 keV of energy (figure 1a). These energetic photons have a high probability of escaping through tissue and can therefore be detected externally.
A PET scanner consists of a large number of detector elements (or a series of large-area position-sensitive detectors) that surround the subject. If two detectors fire within a few nanoseconds of each other, the PET scanner records an “event”. Owing to the collinear nature of the two emitted photons, we immediately know that the site of annihilation lies somewhere in the volume between the two detectors (figure 1b). By collecting large numbers of events (typically 107-108) from many different angles around the subject, we can reconstruct 3-D images. The intensity at any point in the image reflects the concentration of radiotracer in the tissue.
There are two major goals in the design of a PET scanner. The first is to detect as many of the emitted photon pairs as possible to achieve a high signal-to-noise ratio. This ratio is proportional to the square root of the number of events that contribute to the image, so the quality of the PET scan improves as the number of detected events increases. The quality therefore depends on the amount of radioactivity in the tissue, the imaging time and the sensitivity of the scanner.
The second important goal is to localize photon interactions in the detectors as accurately as possible. This determines the spatial resolution, or sharpness, of the images. Two important factors limit the precision with which the location of the decaying nucleus can be determined and therefore the spatial resolution in PET. The first is the distance the positron travels before it annihilates, known as the positron range. For low-energy positron emitters, such as carbon-11 and fluorine-18, the mean range can be as low as a few tenths of a millimetre. For some higher-energy emitters it can be several millimetres.
The second factor is that the electron and positron are not completely at rest when they annihilate. Rather than being emitted back-to-back, the two annihilation photons will be emitted at a slight angle, and this results in a small positioning error, which depends on the separation of the detectors. For a human PET scanner with a detector separation of 80 cm the error is about 2 mm. For these reasons it is probably fair to say that PET in human imaging is unlikely to achieve a spatial resolution much better than 2 mm.
A partial list of radionuclides that decay by positron emission and have been used in biomedical imaging studies.
| Radionuclide | Half-life | Maximum positron energy (MeV) |
| Carbon-11 | 20.1 minutes | 0.96 |
| Nitrogen-13 | 9.96 minutes | 1.19 |
| Oxygen-15 | 123 seconds | 1.72 |
| Fluorine-18 | 110 minutes | 0.64 |
| Copper-64 | 12.6 hours | 0.58 |
| Gallium-68 | 68.3 minutes | 1.9 |
| Bromine-76 | 16.1 hours | 3.7 |
| Rubidium-82 | 78 seconds | 3.35 |
| Iodine-124 | 4.18 days | 1.5 |
PET in the hospital
The first studies to use positron-emitting radionuclides for medical applications were performed back in 1951 by William Sweet at Massachusetts General Hospital and Frank Wrenn at Duke University. In 1974 Michael Phelps, Ed Hoffman and Michael Ter-Pogossian at Washington University in St Louis developed PETT III, the first tomographic device for imaging positron-emitting radionuclides in humans and the forerunner of today’s clinical PET scanners.
PET spread quickly to major medical-research institutions where it was used to study human physiology and metabolism, in healthy subjects and those with a wide range of diseases. A number of clinical applications emerged, primarily based on the radiotracer 18F-fluorodeoxyglucose (FDG). The accumulation of FDG in the body leads to PET images in which the signal intensity is roughly proportional to glucose metabolism.
It turns out that many diseases affect glucose metabolism. In most cancers, for example, there is an enhanced uptake of FDG and this has led to PET being widely used to assess cancer patients, in particular, to check whether the disease has spread to distant sites. Many neurological diseases, including childhood epilepsy and Alzheimer’s, cause specific changes in the distribution of FDG in the brain that can be identified using PET scans.
PET also has important applications in heart disease, where FDG can provide information on the viability of cells in areas of the heart with low blood flow and help determine which patients are likely to benefit from bypass surgery. Indeed, PET has grown rapidly over the past decade, and there are now more than 600 PET scanners worldwide.
The vast majority of clinical PET scanners use scintillation detectors to record the pairs of annihilation photons. The scintillator is segmented into an array of crystals and is read out by a matrix of photomultiplier tubes. Photons interacting in the scintillator produce light that is converted into electrons and amplified by the photomultipliers. The ideal scintillator for PET would be dense, bright and fast, as well as cheap to manufacture and easy to handle.
Historically, bismuth germanate has been the scintillator of choice for PET. Because it is dense, it has a high interaction cross-section and the 511 keV photons can be detected more efficiently. The size of the elements in the scintillator determines the accuracy with which an event can be detected and therefore the spatial resolution of the PET scanner. In a typical device the elements are 3-6 mm across.
Another approach uses large slabs of thallium-doped sodium iodide coupled to a matrix of photomultiplier tubes. The position of the event is calculated from the relative magnitude of the signals in a cluster of photomultipliers surrounding the interaction. Thallium-doped sodium iodide, or NaI(Tl), is used because it is one of the brightest scintillators available and produces large, statistically reliable signals on each of the photomultiplier tubes. The spatial resolution of such detectors is typically about 4-6 mm.
Clinical PET scanners have evolved into sophisticated instruments, with as many as 20 000 scintillation elements multiplexed onto arrays of hundreds of photomultipliers surrounding the patient. They can process and sort hundreds of thousands of events per second. The sensitivity of most PET scanners (i.e. the percentage of photon pairs detected) is between 0.5% and 5%. It is limited primarily by the solid-angle coverage of the detectors that surround the patient, which in turn is limited by cost considerations.
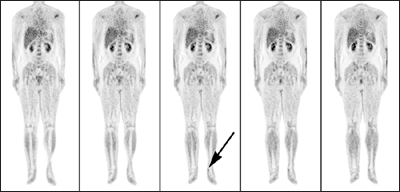
What about the other factors that affect the quality of the final PET images? Patients in clinical imaging studies are usually injected with a 350 megabecquerel dose of radiotracer – equivalent to 350 million nuclear decays per second. This leads to a radiation dose that is similar to that received by patients undergoing other diagnostic imaging procedures, such as X-ray computed tomography. Imaging times vary from a few minutes to 1 hour depending on the imaging protocol. The spatial resolution of the final reconstructed images is limited by the number of collected events, rather than the detector resolution itself. Indeed, in the case of low signal-to-noise measurements, we smooth the images to achieve sufficient diagnostic quality. The typical spatial resolution of clinical PET images is 8-15 mm (figure 2).
PET in the biomedical-research laboratory
Much of the detector research for PET imaging over the past five years has focused on developing scanners for imaging animals. Biologists are creating sophisticated models of human disease in laboratory animals, particularly in the mouse, which shares roughly 95% of its genes with humans. An increasing number of important mouse models are being used to develop new therapeutic approaches for a range of deadly diseases including cancer and Alzheimer’s.
Imaging allows individual animals to be tracked non-invasively over time, which can speed up experiments and reduce the number of animals used in research. Moreover, the same imaging experiments can later be performed in humans, providing an all-important bridge between animal models and the clinic. This should lead to a better understanding of the appropriateness of the animal model in predicting the success of new therapeutic approaches. PET has obvious attractions for these studies because of the wide range of biological targets and processes that can be imaged using the appropriate radiotracer.
The challenge has been to find ways to dramatically improve the image resolution achieved in the clinical setting (8-15 mm), to the level needed to clearly resolve the organs in a mouse (1-2 mm). To achieve such detail requires high-resolution detectors and high-sensitivity systems. If PET is really to become a tool in the biologists’ laboratory, it is also important to develop systems that are quantitative, relatively low cost, compact and easy to use. Our group at the University of California at Davis, along with many other research groups around the world, has taken up this challenge. Aided by a number of important developments in detector technology, several successful PET systems for small animals have now been developed (figure 3).
Early work was carried out at the Hammersmith Hospital in the UK and at Sherbrooke University in Canada in the early 1990s, but breaking the 2 mm resolution barrier required new technology and a different approach. One catalyst was the availability of several new bright and fast scintillators, in particular lutetium oxyorthosilicate (LSO). With an effective atomic number of 66 and a light output that is two-thirds that of sodium iodide, LSO is one of the brightest and most dense scintillators known. These qualities enable small crystals of LSO to detect annihilation photons efficiently for images with a high spatial resolution.
The second technological advance was the dramatic improvement in multichannel and position-sensitive photomultipliers. In 1996 we put these two developments together and assembled detectors from LSO elements measuring 2 mm across mounted on a multichannel photomultiplier. Our prototype scanner – dubbed microPET – consisted of 1920 LSO elements arranged in eight rings around the animal. It produced PET images of living animals that broke the 2 mm resolution barrier for the first time (figure 4). At about the same time, Simone Weber from Jülich University in Germany and Alberto Del Guerra from Ferrara University in Italy developed animal PET systems with 2 mm resolution based on another new scintillator, yttrium perovskite. More recently Michael Green and colleagues at the US National Institutes of Health in Maryland have built a 2 mm resolution scanner based on layers of LSO and a closely related scintillator, gadolinium oxyorthosilicate, leading to higher-efficiency detectors. Meanwhile, the original microPET scanner has been commercialized by Concorde Microsystems in Tennessee, and more than 25 institutions have now taken delivery of these animal PET systems.
These new scanners have opened up many opportunities for research into animal models, but the spatial resolution is still too low to visualize important substructures in the mouse brain, the earliest manifestations of tumours or the spread of cancer to other organs. Three different groups are currently pursuing combinations of sub-millimetre LSO detector elements coupled to new-generation multichannel and position-sensitive photomultiplier tubes. Jack Correia and co-workers at Massachusetts General Hospital have built a PET scanner based on 1-D detector arrays that produce images with a resolution of 1 mm. Unfortunately, the device can currently only image single slices of an object, rather than the entire volume. Meanwhile, Robert Miyaoka at the University of Washington in Seattle has successfully built 2-D detector arrays that have a resolution of 1 mm. And our group has just completed the next-generation microPET scanner, which has 17 640 LSO detector elements measuring 0.95 x 0.95 mm, coupled to 90 multichannel photomultipliers.
The resolution of these new systems will soon hit some of the limitations due to the physics of positron annihilation. Moreover, the new detectors are likely to need sophisticated image-reconstruction algorithms that accurately model all the factors that degrade spatial resolution.
Alternative technologies
There are not many fields of science where vacuum tubes are still the technology of choice. Yet photomultiplier tubes have enjoyed extraordinary longevity in PET because of their high gain, low noise and fast response. For many years researchers have been attempting to develop solid-state photon detectors that have reasonable gain and high sensitivity in the blue part of the spectrum (which is where most scintillators emit their light), and that are fast enough for applications in PET.
Finally, after years of development, arrays of silicon “avalanche photodiode detectors” (APDs) have become commercially available and are finding their way into animal PET scanners. Photons emitted from the scintillator generate an electron-hole pair in the APD. The applied electric field (as high as 106 V m-1) accelerates the electron sufficiently to create further electron-hole pairs, leading to an avalanche effect. Internal gains between 50 and 1000 have been achieved depending on the structure of the device. A prototype animal PET scanner based on LSO scintillators and APDs has been constructed by Sybille Ziegler and Bernd Pichler at the Technical University of Munich. Meanwhile, a group known as the Crystal Clear Collaboration at CERN in Geneva is developing APD-based devices and will be working with three European universities to build small-animal PET scanners.
Almost all the animal PET scanners built so far have used scintillation detectors because of their high efficiency at detecting 511 keV photons. However, a very different approach has been developed by Alan Jeavons at Oxford Positron Systems in the UK using multiwire proportional chambers, which were first developed for use in particle physics. This system consists of four large detectors, each containing a stack of wire chambers interleaved with a sandwich of thin lead plates that are drilled with a fine matrix of holes. The lead plates convert a small fraction of the incident annihilation photons into electrons while the wire chambers determine the position of the charge. While these detectors can provide sub-millimetre resolution, they have a much lower efficiency per unit area compared with scintillation detectors. This will ultimately limit the sensitivity of PET scanners based on this technology.
It is exciting to see so many different detector approaches being developed and used for small-animal PET imaging. It is even more exciting to see the array of applications that these high-performance imaging systems have opened up, independent of the specific technology. These applications include imaging specific receptor systems in mouse and rat brains, imaging the effectiveness of new anti-cancer agents in mouse models, and studies into the effect of brain injury, such as sports-related trauma, on brain function.
Recently, Sam Gambhir and co-workers at the University of California in Los Angeles and Ron Blasberg’s group at Sloan-Kettering Hospital in New York successfully merged the techniques of molecular biology and radiotracer development to track specific genes in living animals using PET. In particular they have been able to follow how these genes are “expressed” or translated into proteins within the body over time (figure 4). This important work takes us one step closer to using PET to monitor the effectiveness of new gene therapies for humans, in which missing or “under-expressed” genes are introduced into the body.
Molecular imaging meets anatomic imaging
PET radiotracers accumulate wherever they encounter the biomolecules or the biochemical pathways with which they are designed to interact. In general, the uptake of radiotracers that have been tailored towards highly specific reactions is restricted to very few regions in the body. On a PET image these regions just appear as shapes with little or no anatomical information, making it difficult to interpret the scan. For example, researchers find it hard to tell if the radioactivity seen around the prostate region of a mouse is due to a specific accumulation in cancer cells or just a build-up of radiotracer that has been flushed into the bladder. Is an accumulation of radiotracer in the chest really in the lung tissue or is it in the rib bone? The lack of anatomical information is a problem both in human and in small-animal PET studies.
Software approaches for combining data from different imaging techniques have been around for many years and have become increasingly sophisticated. These work well in the brain because it is highly constrained by the skull, making it relatively straightforward to overlay two image data sets precisely. However, this approach becomes very difficult in the rest of the body because organs move about and change shape depending on how the patient lies in the scanner. Things also change over time – for example the bladder changes shape as it fills with urine and causes surrounding tissues and organs to move.
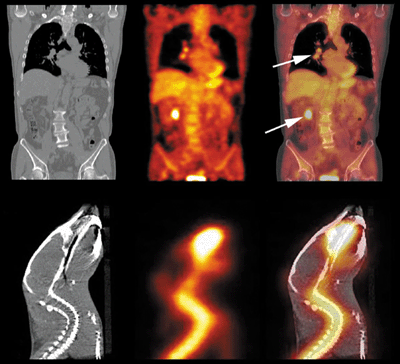
One of the most important developments in recent years has therefore been the development of “multimodality” imaging systems, in which PET scans and high-resolution anatomic images are acquired at essentially the same time in a single device. The most successful integration has been PET with X-ray computed tomography (CT), an idea developed by David Townsend and colleagues at the University of Pittsburgh in collaboration with CTI PET Systems in Knoxville.
Townsend’s group placed a clinical PET detector in tandem with a clinical CT scanner around a patient bed. The new device allowed PET and CT images to be obtained in quick succession and overlaid with almost perfect correlation (figure 5a). Combined PET/CT scanners are now available from a number of manufacturers and are having a huge clinical impact. Any suspicious anatomical abnormality seen on the CT scan can now be correlated with the metabolic activity of that region from the PET scan. A high level of activity indicates a malignant cancer, while a low level implies a more benign process.
Our group at Davis has been exploring PET/CT imaging for small-animal studies. We have combined our microPET detector technology with flat-panel amorphous selenium X-ray detectors, which were originally developed for digital mammography. In this case the system is coplanar, meaning that the same region is scanned simultaneously with PET and CT (figure 5b). The low-energy X-rays suitable for imaging small animals can easily be separated from 511 keV annihilation photons in the detector.
Work is also under way to develop combined PET and magnetic resonance imaging techniques. Magnetic resonance imaging has several advantages over CT scanning – it provides better contrast in soft tissue and is also capable of functional and spectroscopic measurements. However, integrating these two technologies is much more challenging owing to the high magnetic fields used in scanners for magnetic resonance imaging (up to 10 T), and the need for high field uniformity (1 part in 106).
Nonetheless our group at Davis, together with Paul Marsden at Kings College, London, has started investigating PET imaging systems that can be placed inside the bore of conventional scanners for magnetic resonance imaging. Successful prototypes have been developed that use optical fibres to pipe the scintillation light from a ring of LSO elements inside the magnet to photomultiplier tubes and electronics 3 metres away. This distance minimizes any problematic interactions between the magnetic field and the photodetectors and electronics. The first studies of animals in which PET and magnetic resonance images are acquired simultaneously should be under way by the end of the year.
A bright future for PET
Recent advances in PET-detector technology have resulted in high-performance imaging systems that enable PET to be used from mouse to man. This provides a critical bridge between mouse models of disease – where new therapeutic approaches will be developed – and the clinic, where these therapies will ultimately be applied. The future appears to be very bright.
PET could be used to non-invasively and efficiently assess new drugs in animal models, and may play a role in selecting the drugs that should move forward into clinical trials. In humans, PET could help to diagnose molecular or genetic diseases. It could also be used to assess whether a very low and safe quantity of a drug reaches its target in sufficient concentration to exert the desired biological effects. PET imaging might even be used to select patients who will respond to a particular drug, and could monitor the direct biological effects as the patient is treated.
There is a long way to go before we can put these approaches together, but the technology now exists to undertake these types of studies. Perhaps the clearest sign of the potential role of PET in preclinical and clinical medicine is that virtually all the major pharmaceutical companies are collaborating with academic PET centres. Several of these companies are even setting up their own PET-imaging facilities.
Probably the biggest single challenge now is in radiotracer design. There are many biological targets of interest for which we do not yet have good radiotracers, and many others where novel radiotracers await proper characterization. We can also look forward to further improvements in the resolution and sensitivity of PET scanners – for both humans and animals – and perhaps significant reductions in the cost of these systems.
All these improvements will almost certainly be driven by new technologies – such as better scintillators, new photodetectors and dense semiconductor materials – and these will most likely be discovered and developed by the physics community. Indeed, there has never been a better time for physicists to work with chemists and biologists to build the tools and techniques needed to translate our growing understanding of biology into safe and effective new treatments for disease.