
The noble gas xenon reacts with water ice at exceedingly high temperatures and pressures – conditions that are found within the interiors of planets such as Uranus and Neptune. That’s the conclusion of an international team of researchers who used X-ray diffraction and theoretical calculations to determine the crystal structure of the resulting compound, which has a Xe4O12H12 primitive cell. The findings take planetary scientists one step closer to solving the “missing xenon paradox” and could also improve our understanding of xenon’s isotopes.
In the early 1970s researchers noted the surprising lack of xenon in Earth’s atmosphere when compared with the concentration of other noble gases – almost 90% of the expected amount of xenon is missing. However, xenon is found at the expected abundance elsewhere in the solar system – on meteorites, for example. This suggests that the mysteriously missing noble gas is merely hiding somewhere on our planet.
Curiouser and curiouser
A multitude of theories have been purported suggesting that, for example, xenon might have been ejected into space, or be trapped on Earth in the polar caps, or stuck in sediments, deep in oceanic trenches or even within the Earth’s core. But none of the theories could account for all of the missing gas. Further research has also found that both Mars and Jupiter seem to have a similar lack of xenon within their atmospheres.
As a noble gas, xenon is assumed to be non-reactive under normal conditions. But over the years, researchers have tried to make chemical compounds containing xenon at extreme pressures and temperatures similar to those that are found deep within the Earth. In 1997 scientists tried to react xenon with iron under such conditions, but found that no compound was formed. In 2005 Chrystele Sanloup of Pierre and Marie Curie University in Paris, along with colleagues, found that the gas could displace and then substitute silicon in quartz at high temperatures and pressures. However, the researchers also noted that the xenon escapes just as easily from the material. Further work, carried out by another group, found that xenon could also bond to oxygen within quartz, allowing the researchers to synthesize xenon dioxide (XeO2) for the first time.
Extreme measures
Sanloup (now at the University of Edinburgh) and colleagues in France, the UK and US have now shown that at pressures above 50 GPa and a temperature of 1500 K, xenon reacts with water ice to form Xe4O12H12. Sanloup used a diamond-anvil cell – a device that squeezes a sample between two tiny, gem-grade diamond crystals. This was laser heated to create extreme conditions similar to those in the interior of the “ice-giant” planets Uranus and Neptune. Currently, the atmospheres of Uranus and Neptune have not yet been probed for their xenon concentrations.
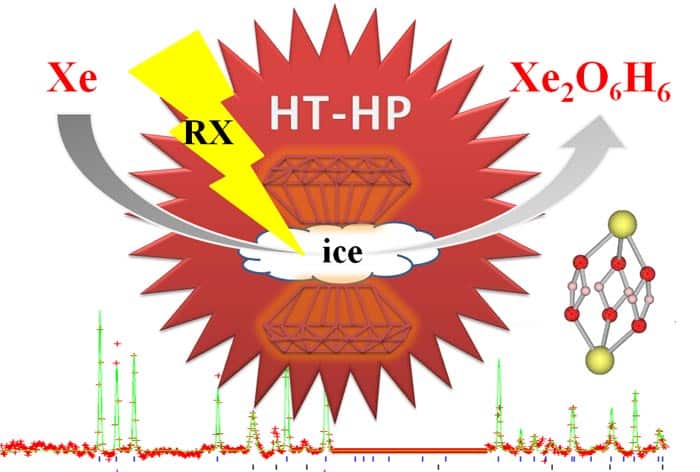
Sanloup carried out her experiments on the ID27 beam line of the European Synchrotron Radiation Facility (ESRF) in Grenoble, France. “Once we were above 50 GPa, we could see a reaction systematically taking place and could see a distinct diffraction pattern that suggested a new phase [was formed],” she says. Sanloup went on to tell physicsworld.com that the structure that best describes the new phase is a hexagonal lattice with four xenon atoms per unit cell. However, there were several possible distributions of the oxygen atoms.
Atom build-up
Sanloup then roped in Stanimir Bonev from the Lawrence Livermore National Laboratory in the US to analyse the structure and resolve the location of the oxygen atoms. It was during this analytical work that the team found that none of the solutions with only oxygen worked, so hydrogen was added to “build up” the final Xe4O12H12 structure. “The hydrogen would not have been seen with the X-ray diffraction as it would react too lightly,” explains Sanloup. “But in the future we could use Raman spectroscopy to see it or we could use neutron diffraction instead of X-rays, but for that we would need a much larger sample,” she says. The team suggests that its newly discovered compound has a weakly metallic character and could be formed in superionic ice – a phase of water that is believed to exist at high pressures and temperatures.
Sanloup explains that solving the mystery of the missing xenon is crucial because the relative abundances of radioactive xenon isotopes are widely used by geochemists as a tool to probe major terrestrial processes, such as when the Earth’s atmosphere formed. Naturally occurring xenon has eight stable isotopes and more than 40 unstable isotopes that undergo radioactive decay. Isotope ratios are also used to study the early history of our solar system, including to model planet formation. But most of these calculations assume that xenon is mostly non-reactive. The new findings could alter our knowledge of the xenon isotopes, which in turn would affect our models of planet formation and evolution.
The research is published in Physical Review Letters.


