Cyber attacks on hospitals can have a devastating impact, especially for radiology and radiotherapy departments that are particularly reliant upon technology to function. A case in point is the nationwide cyber attack on Ireland’s public health services in May 2021, which interrupted scheduled radiotherapy treatments for some cancer patients for up to 12 days.
Following this incident, medical physicists at University Hospital Galway and the National University of Ireland Galway began to develop an in-house tool to help create revised radiotherapy treatment plans after interruptions occur. The tool – named EQD2VH – calculates treatment compensation plans and enables visual comparison of all plan options, as well as individual analysis of each structure in a patient’s plan. The researchers describe the new software tool in the Journal of Applied Clinical Medical Physics.
Radiotherapy is most commonly delivered over several weeks in a series of small radiation doses (conventionally 2 Gy) called fractions. Unplanned treatment gaps – whether due to cyber attacks, machinery breakdowns or patient illness – can cause significant setbacks. During such gaps, cancer cells rapidly repopulate in tumour tissue, resulting in a decrease in the radiobiological dose to the planning target volume (PTV).

To address this problem, EQD2VH uses dose–volume histogram (DVH) information extracted from original patient plans to perform treatment gap calculations. Lead author Katie O’Shea, of the National University of Ireland Galway, and colleagues explain that the software converts the physical dose in each dose bin (the range of dose between data points in a DVH) into the biologically effective dose (BED). This accounts for both repopulation effects in the PTV and the effects of sub-lethal damage to unrepaired normal tissue in organs-at-risk (OARs).
After modifying the BED conversion to account for dose variations in each structure, using a variable-dose method, the tool converts the BED for each structure into the equivalent dose in 2 Gy fractions (EQD2). This normalizes each treatment to conventional fractionation and makes it possible to sum plans with different fractionation schemes together. The resultant EQD2 -based DVH provides a 2D representation of the impact of treatment gap compensation strategies on both PTV and OAR dose distributions, as compared with the prescribed treatment plan.
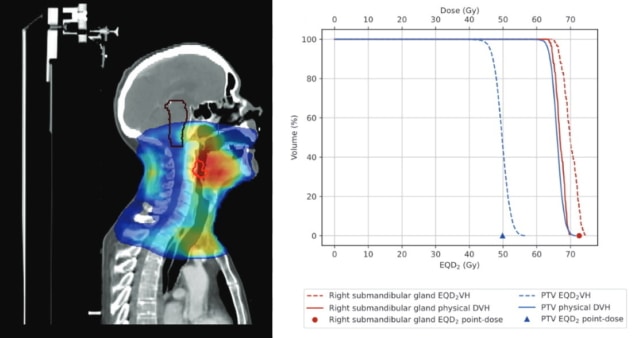
To evaluate EQD2VH as a clinical decision-making tool, the researchers selected five high-priority patients with rapidly growing tumours whose treatment gaps should not surpass two days. This included four patients with head-and-neck cancers undergoing intensity-modulated radiotherapy and one lung cancer patient undergoing 3D conformal radiotherapy, who had treatment gaps of 12 or 13 days. These cases enabled the team to evaluate the use of EQD2VH for patients with both conventional (2 Gy) and non-conventional (2.2 Gy) fractionation and different treatment gap times (from nine to 46 days into their therapy).
The revised treatment plans for each patient were based on their original plans with either the dose-per-fraction or the number of fractions changed. O’Shea explains that each patient’s revised plan and schedule used a combination of twice-daily fractionation, weekend treatments and increased dose to the target volume to reduce the effects of cell repopulation.
The plans limited treatment to six fractions per week and precluded twice-daily fractionation on consecutive days. If the prescribed treatment could not be completed in the required time frame, the researchers investigated plans using hypofractionation (delivery of increased dose per fraction). They were able to visually and quantitatively compare various revised plans with the patient’s original plan to determine which would deliver the best dose to the PTV with the least dose to OARs.
The researchers note that the 2D representation of each individual structure in EQD2VH provides a more in-depth analysis than the Royal College of Radiologists (RCR)-recommended 1D point-dose calculation method that’s currently used to manage radiotherapy gaps. A 1D representation of dose distribution within a volume does not account for OARs typically having a non-uniform dose distribution and could overestimate OAR dose. In addition, the EQD2VH tool can create plans for any length of treatment gap, whereas the RCR guidelines are based on a standard gap of four to five days.
Additional benefits of the new tool include the ability to monitor each OAR in the patient’s plan to minimize further dose increases that could cause more acute toxicities. Users can also calculate the impact of different treatment gap durations on a patient’s treatment. This capability can help determine whether to transfer a patient to a different clinic if the gap at the scheduled clinic is too long or whether the patient can safely wait for treatment to resume.

How has COVID-19 impacted the provision of radiation therapy?
EQD2VH can also account for changes in the overall treatment time and sublethal damage in normal tissue, which a commercial system may not be able to do. Most importantly, the tool does not need to be connected to hospital network to function – it can be used even if a hospital’s servers are still crippled by a cyber attack.
“We are still evaluating EQD2VH as a decision-making tool,” says principal investigator Margaret Moore from University Hospital Galway. “It is part of a current project reviewing patients receiving multiple re-treatments for palliative regimes where the dose-per-fraction is non-standard and where there may be a choice of fractionation schemes to consider. Converting treatment dose from a number of treatments with differing fractionations to EQD2 allows the radiobiological dose to target tissues and OARs to be accumulated for an overall dose overview, which can assist with the decision-making for the choice of further treatment.”



