Researchers at Duke University Medical Center have developed a deep-learning-based computer-aided detection (CAD) system to identify difficult-to-detect brain metastases on MR images. The algorithm exhibited excellent sensitivity and specificity, outperforming other CAD systems in development. The tool shows potential to enable earlier identification of emerging brain metastases, allowing them to be targeted with stereotactic radiosurgery (SRS) when they first appear and, for some patients, reducing the number of required treatments.
SRS, which uses precisely focused photon beams to deliver a high dose of radiation to targets in the brain in a single radiotherapy session, is evolving into the standard-of-care treatment for patients with a limited number of brain metastases. To target a metastasis, however, it must first be identified on an MR image. Unfortunately, approximately 10% are not, 30% for those less than 3 mm in size, even when reviewed by expert neuroradiologists.
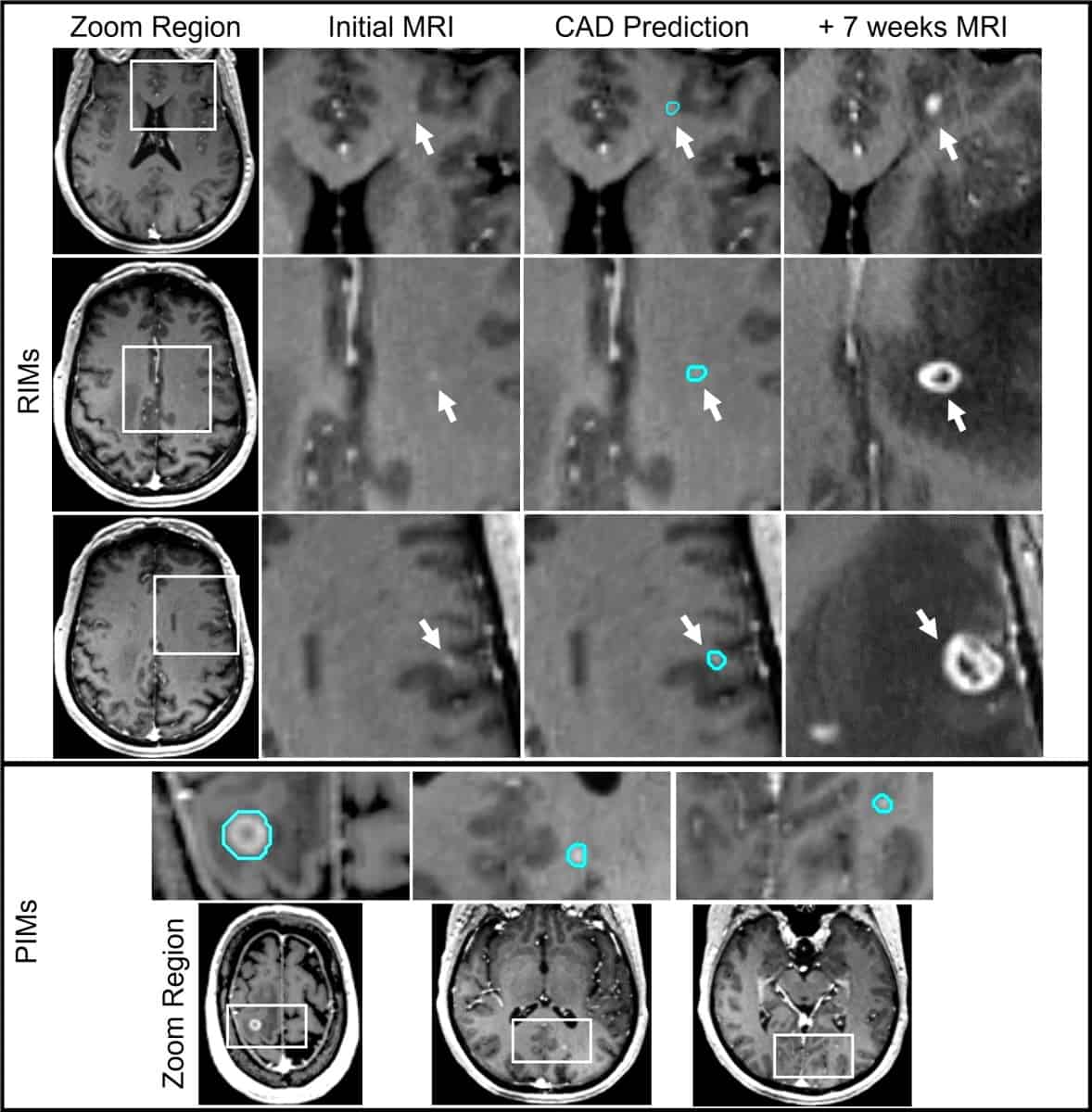
When these undiscovered brain metastases – which the researchers refer to as retrospectively identified metastases (RIMs) – are identified on subsequent MRI scans, a second SRS treatment is usually needed. Such treatment is expensive, and can be uncomfortable and invasive, sometimes requiring head immobilization with a frame secured to the skull by pins.
At the recent ASTRO Annual Meeting, Devon Godfrey explained that the researchers designed the convolutional neural network (CNN)-based CAD system specifically to improve the detection and segmentation of hard-to-detect RIMs and very small prospectively identified metastases (PIMs). Godfrey and colleagues describe the testing and validation of this system in the International Journal of Radiation Oncology Biology Physics.
The team trained the CAD tool on MRI data (a contrast-enhanced spoiled gradient echo sequence) from 135 patients with 563 brain metastases. The images were acquired using 1.5 T and 3.0 T MRI scanners from different vendors at multiple Duke Health locations. In total, the data set included 491 PIMs with a median diameter of 6.7 mm, and 72 RIMs from 32 patients, with a median diameter of 2.7 mm.
To identify RIMs, the researchers reviewed each patient’s original MR images to search for signs of contrast enhancement in the exact location where a metastasis was later detected. After review, they classified each RIM as either having met imaging-based diagnostic criteria (+DC) or having insufficient visual information (-DC) to be identified as a metastasis.
The researchers randomized the data set of RIMs and PIMs into five groups, using four of these for model and algorithm development and one as a test group. “The inclusion of both +DC and -DC RIMs resulted in the highest sensitivities for every brain metastasis category and size, while also returning the lowest false-positive rate and the highest positive predictive value,” they report. “This shows a clear benefit of including an overweighted sampling of small challenging brain metastases to CAD training data.”
For PIMs and +DC RIMs – which have clear characteristics of metastases on MRI – the model achieved an overall sensitivity of 93%, ranging from 100% for lesions larger than 6 mm in diameter to 79% for those smaller than 3 mm. The false-positive rate was also impressively low, with a mean of 2.7 per person, compared with between eight and 35 in other CAD systems with comparable detection sensitivity for small lesions.
The CAD system was also able to detect some of the -DC RIMs in both the development and test sets. Identification of brain metastases at this earliest stage would be a great clinical advantage, as such lesions could then be more thoroughly monitored with imaging, prompting treatment if required.
The Duke team is now working to improve the CAD tool’s accuracy by utilizing multiple MR sequences. Godfrey explains that brain MRI studies almost always include multiple MR sequences that produce unique information about every voxel in the brain. “We believe that incorporating the additional information available from these other sequences ought to improve its accuracy,” he says.
Godfrey notes that the researchers are just weeks away from launching a simulated prospective clinical use study of the existing CAD system to investigate how the tool impacts clinical decision making by both radiologists and radiation oncologists.
Deep learning helps radiologists detect lung cancer on chest X-rays
“Multiple expert neuroradiologists and neuro-radiation oncologists who perform SRS will be presented with brain MR scans. They will be asked to find any lesion that might be a brain metastasis, rate their confidence level that it is, and state whether they would treat the lesion with SRS, based upon its appearance in the images,” he tells Physics World. “We will then present them with the CAD predictions and evaluate the impact of CAD on each physician’s clinical decisions.”
If this simulation study yields promising results, Godfrey anticipates deploying the CAD tool to help identify challenging brain metastases prospectively in new patients being treated in the Duke Radiation Oncology clinic under a research protocol, perhaps as soon as mid-year 2023.



