Magnetic detection and manipulation of cells is attractive for numerous biomedical applications, and so scientists have been eager to discover how many types of fish, amphibians, mammals and birds can sense changes in the earth’s magnetic field. Some theories claim that this “magnetoreception” is driven by biomagnetic structures – but these have yet to be found. Taking a complementary approach, a team of researchers led by Gil Westmeyer at the Technical University of Munich and Helmholtz Zentrum München have bioengineered a magnetoresponsive system using bacterial components (Nature Comm. 9 1990).
The researchers expressed bacterial shell proteins called encapsulins within mammalian cells and showed that they self-assemble into enclosed nanospheres that can accumulate substantial amounts of iron in their interior. The researchers were thus able to magnetically sort and separate the cells containing iron-rich nanospheres from non-magnetic cells, and use them to generate contrast in MRI and act as a genetic marker for electron microscopy.
In addition to applications with iron-accumulating nanocapsules, Westmeyer’s team explored the encapsulation of different types of cargo, such as enzymes, turning the nanospheres into mini bioengineering “workshops” within eukaryotic cells. “This method to genetically control compartmentalization of multi-component processes will be quite useful as a general tool for mammalian cell engineering,” says Westmeyer.
Building an iron accumulation chamber
Firstly, the researchers modified encapsulin genes from the bacterium Myxococcus xanthus and added them into a mammalian cell line, forcing expression of the nanospheres. A variety of identifying tags were added to the encapsulin shell protein (EncA) and its native cargo proteins so that the expression, self-assembly and cargo loading could be verified in in vitro assays. They also employed cell viability assays and showed encapsulins to be non-toxic.
Next, the researchers moved encapsulins in vivo, encoding them in adeno-associated viruses that they intracranially injected into mice. The nanospheres were robustly expressed and assembled in neurons, and again showed no toxic effects.
Native encapsulin cargo proteins EncB and C have iron storage capabilities, and when expressed with EncA, were able to accumulate and shield iron within the nanospheres from the metabolic processes occurring within the cytosol of a mammalian cell.
Magnetic genetics
By creating these iron accumulation chambers within mammalian cells, the researchers were able to significantly enhance the MRI contrast of cells, and Westmeyer hopes that, in the future, iron loaded encapsulins could possibly be used for deep-tissue molecular imaging and manipulation. For instance, optogenetics is a technique that uses light to control molecular processes in living animals; the drawback is that light does not travel well through tissue. Magnetic fields, on the other hand, could be used for molecular manipulation deeper in tissue. “With encapsulins we have started to obtain sufficiently magnetic handles that are genetically controlled,” Westmeyer explains.
The researchers also demonstrated that encapsulins could be used as a genetic marker in electron microscopy. Chemical reagents are often used to obtain contrast from molecular signals in electron microscopy, but the process of delivering these reagents can disrupt intracellular structures and complicates more high-throughput applications.
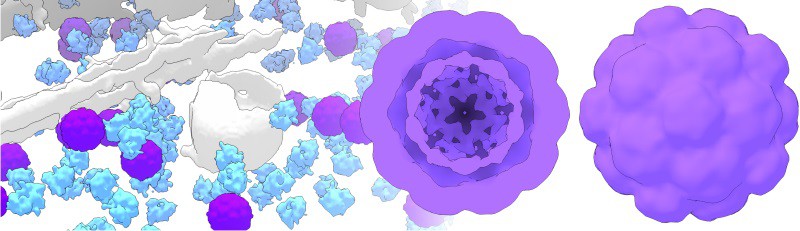
“Encapsulins possess a well-defined structure that is clearly discernible in cryo-electron microscopy, and when they accumulate iron they exhibit additional contrast without extra preparative steps involving synthetic compounds,” says Westmeyer. As a direct, fully genetically encoded reporter, Westmeyer hopes encapsulins could generate similar value for electron microscopy as green fluorescent protein and its powerful variants delivered for fluorescent microscopy.
A workshop for bioengineering
In this study, the researchers also showcased the encapsulation of other, non-native proteins into the nanospheres. By simply adding an eight amino acid targeting tag, they were able to isolate specific enzyme reactions within the nanospheres, recruiting small reactants through pores in the shell but shielding enzymes and products from the cell’s complex metabolic processes.
Mammalian cell engineering is often confounded by the complexity of molecular processes, but Westmeyer thinks that the capability to contain reactions within encapsulins and prevent interference from other cellular processes will help the field to advance.
“The 32 nm nanospheres are tiny workshops, subspaces where we control which molecular players act on the inside… and things can be quite different from the office next door (the cytosol),” says Westmeyer. “Our next steps will be to exploit the modularity of these genetically controlled compartments to install more interesting functionalities into mammalian cells.”