A research collaboration between Dutch radiotherapy clinic Maastro and independent QA specialist CIRS suggests it’s time to rethink best practice regarding the dosimetric verification of stereotactic treatment plans for very small lung tumours
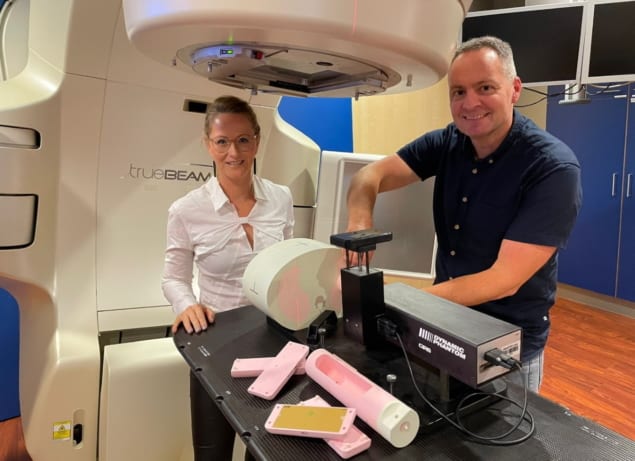
Medical physicists at the Dutch radiation oncology clinic Maastro are on a mission to fast-track continuous improvement and best practice in the planning, management and delivery of stereotactic body radiotherapy (SBRT) for the treatment of very small (less than 1 cm diameter) early-stage lung tumours. Working with industry partner CIRS, a US manufacturer of tissue-equivalent phantoms and simulators for medical imaging, radiation therapy and procedural training, Michel Öllers, Ans Swinnen and colleagues set out last year to demonstrate the inadequacy of existing dose verification methods for a specific category of lung SBRT treatments (Med. Phys. 47 5829). Their focus: how the latest so-called “type c” dose calculation algorithms like Acuros, an integral part of Varian’s Eclipse treatment planning system (TPS), deviate beyond a relative uncertainty of 5% for small lung tumours.
“Modern dose calculation algorithms perform much better in heterogeneous tissue like the lung than they did, say, 15 years ago,” explains Frank Verhaegen, head of physics research at Maastro and professor of medical physics at Maastricht University. “Nevertheless,” he adds, “accurate dose verification and QA for treatment plans of small lung tumours – and therefore small treatment fields – remains a complex proposition for the medical physics team.”
Phantom physics
Part of the problem is that while water-equivalent phantoms are well suited to the QA of radiotherapy treatments for abdominal or brain tumours (where most of the tissue is near-water-equivalent), the use of those same water-equivalent phantoms for heterogeneous tissue (such as the microenvironment of the lung) will provide incorrect dosimetric results. “Our aim was to find a phantom suited for accurate dose verification of the very small lung tumours (below 1 cm3) that are increasingly treated nowadays using SBRT,” notes Öllers. “Those cases, in turn, represent a non-trivial physics challenge for the developers of any dose calculation algorithm.”
With this in mind, the Maastro researchers teamed up with product engineers at CIRS to co-develop a series of proprietary tumour-equivalent inserts that mimic lung-tissue lesions as small as 5 mm diameter when integrated within CIRS’s existing Dynamic Thorax Phantom (Model 008A). Their new heterogeneous set-up was subsequently put through its paces at Maastro to test the premise, as Verhaegen puts it, that “accurate plan verification of small lung tumours can only be performed in an anthropomorphic phantom that mimics the clinical situation as closely as possible”.
Put another way: although the traditional SBRT QA consensus states that type c algorithms are able to reproduce the actual physical dose distribution in heterogeneous media in a small-field setting, Maastro’s working hypothesis said otherwise. In fact, the starting point for Verhaegen and colleagues was just the opposite – an assertion that Acuros falls outside a clinically acceptable accuracy of 5% below a certain threshold in tumour diameter (approximately 0.75 cm).
Quantifying the problem
To test their hypothesis, the Maastro physicists carried out a series of comparative dose measurements using the homogeneous PTW Octavius 4D phantom (including the Octavius 1000 SRS detector) and the heterogeneous Dynamic Thorax Phantom from CIRS. The latter contained different lung-equivalent, film-holding cylindrical phantom inserts with water-equivalent spherical targets (diameters of 0.5, 0.75, 1, 2 and 3 cm).
Using Acuros (version 15.5.11), the team calculated plans for 6 and 10 MV for each spherical target in the CIRS phantom – 14 treatment plans in all – with those plans subsequently delivered to both the PTW and CIRS phantoms to compare measured dose. In addition, the treatment plans of seven clinical lung cancer patients, all of them with tumours less than approximately 1 cm3 by volume, were irradiated in the heterogeneous CIRS phantom. The actual tumour size within the clinical treatment plans determined the choice of the spherical target size, thereby ensuring that both measurement geometry and clinical target volumes match as closely as possible.
The results are unequivocal: while measurement discrepancies in the homogeneous Octavius 4D phantom for the 14 calculated treatment plans were within 1.5%, dose discrepancies between measurement and TPS for the heterogeneous CIRS phantom increased for both 6 and 10 MV plans with decreasing target diameters – to 23.7±1.0% for 6 MV and 8.8±1.1% for 10 MV using the smallest target of 0.5 cm diameter (with a 2 mm margin for planning target volume versus clinical target volume). For the seven clinical plans, meanwhile, this trend of increasing dose difference with decreasing tumour size is less pronounced, although the smallest tumours exhibit the largest differences between measurement and TPS (up to 16.6±0.9%).
Disseminate and educate
So what’s changed 12 months on from the publication of Maastro’s findings? Verhaegen, for his part, is hopeful that the wider radiotherapy community is now at least more aware of the limitations of even the most advanced dose calculation algorithms for challenging SBRT indications like small lung tumours, also of the need for dedicated heterogeneous phantoms that can mimic these clinical situations to provide state-of-the-art dose verification. “Equally important,” he explains, “those heterogeneous phantoms are essential for the testing, calibration and validation of next-generation dose calculation models for stereotactic lung treatments.”
Meanwhile, the Maastro medical physics team has actioned the results with the clinic’s radiation oncologists, who are now aware of the limitations and inaccuracies of SBRT treatment plans to address lung tumour volumes of 1 cm3 or smaller. “Depending on the clinical status of the patient, we might accept dose inaccuracies up to 10% or make a decision not to treat,” says Öllers. “It’s worth noting, though, that our decision to treat or not to treat at Maastro is weighted against several factors. We consider not only the challenges of small lung tumours for the dose calculation algorithm, but also tumour movement and the visibility of the tumour targets on the cone-beam CT image.”
As for the bigger picture, Verhaegen reckons Varian’s close interest in the results is good news for the longer-term development and enhancement of lung SBRT QA protocols. He concludes: “We have been in contact with Varian since the publication of the study and aim to work with the vendor’s Eclipse development team to improve the Acuros algorithm for small lung lesions. That sort of continuous improvement will yield transferable upsides for the radiotherapy community as a whole.”
Phantom dynamics
The CIRS Dynamic Thorax Phantom (Model 008A) is designed for comprehensive analysis and QA of image acquisition, treatment planning and dose delivery in image-guided radiation therapy of lung lesions.
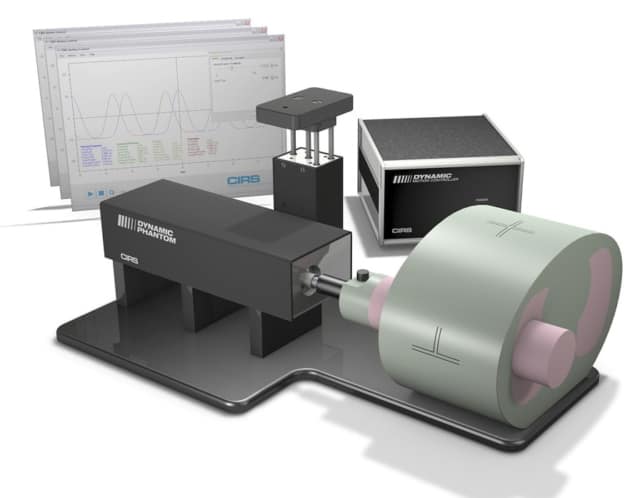
The phantom body represents an average human thorax in shape, proportion and composition. A lung-equivalent rod (containing a spherical target and/or various detectors) is inserted into the lung-equivalent lobe of the phantom.
The body is connected to a motion actuator box that induces 3D target motion (sub-mm accuracy and reproducibility) through linear translation and rotation of the lung-equivalent rod. The motion of the rod itself is radiographically invisible (owing to its matching density with the surrounding material), while the motion of the target can be resolved thanks to its density difference.
CIRS Motion Control Software provides independent control of target and surrogate, replicating the complex 3D tumour motion within the lung. The phantom is tissue-equivalent from 50 keV to 125 MeV, while the surrogate breathing platform accommodates numerous gating devices.




